Alpha-crystallin binds to the aggregation-prone molten-globule state of alkaline protease: implications for preventing irreversible thermal denaturation
- PMID: 12070325
- PMCID: PMC2384148
- DOI: 10.1110/ps.0201802
Alpha-crystallin binds to the aggregation-prone molten-globule state of alkaline protease: implications for preventing irreversible thermal denaturation
Abstract
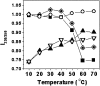
Alpha-crystallin, the major eye-lens protein with sequence homology with heat-shock proteins (HSPs), acts like a molecular chaperone by suppressing the aggregation of damaged crystallins and proteins. To gain more insight into its chaperoning ability, we used a protease as the model system that is known to require a propeptide (intramolecular chaperone) for its proper folding. The protease ("N" state) from Conidiobolus macrosporus (NCIM 1298) unfolds at pH 2.0 ("U" state) through a partially unfolded "I" state at pH 3.5 that undergoes transition to a molten globule-(MG) like "I(A)" state in the presence of 0.5 M sodium sulfate. The thermally-stressed I(A) state showed complete loss of structure and was prone to aggregation. Alpha-crystallin was able to bind to this state and suppress its aggregation, thereby preventing irreversible denaturation of the enzyme. The alpha-crystallin-bound I(A) state exhibited native-like secondary and tertiary structure showing the interaction of alpha-crystallin with the MG state of the protease. 8-Anilinonaphthalene sulphonate (ANS) binding studies revealed the involvement of hydrophobic interactions in the formation of the complex of alpha-crystallin and protease. Refolding of acid-denatured protease by dilution to pH 7.5 resulted in aggregation of the protein. Unfolding of the protease in the presence of alpha-crystallin and its subsequent refolding resulted in the generation of a near-native intermediate with partial secondary and tertiary structure. Our studies represent the first report of involvement of a molecular chaperone-like alpha-crystallin in the unfolding and refolding of a protease. Alpha-crystallin blocks the unfavorable pathways that lead to irreversible denaturation of the alkaline protease and keeps it in a near-native, folding-competent intermediate state.
Figures
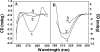

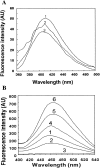
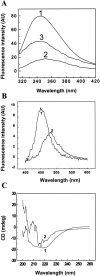
 ), pH 5.0 (▪), pH 3.5 (), pH 2.0 (▴), and pH 3.5 in the presence of 0.5 M Na2SO4 (○).
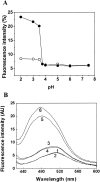
), pH 5.0 (▪), pH 3.5 (), pH 2.0 (▴), and pH 3.5 in the presence of 0.5 M Na2SO4 (○).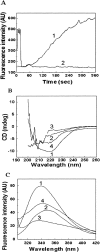
Similar articles
-
Illustration of HIV-1 protease folding through a molten-globule-like intermediate using an experimental model that implicates alpha-crystallin and calcium ions.Biochemistry. 2005 Mar 15;44(10):3725-34. doi: 10.1021/bi048378n. Biochemistry. 2005. PMID: 15751949
-
Unfolding and refolding of a quinone oxidoreductase: alpha-crystallin, a molecular chaperone, assists its reactivation.Biochem J. 2001 Nov 1;359(Pt 3):547-56. doi: 10.1042/0264-6021:3590547. Biochem J. 2001. PMID: 11672428 Free PMC article.
-
Association of partially folded lens betaB2-crystallins with the alpha-crystallin molecular chaperone.Biochem J. 2008 Feb 1;409(3):691-9. doi: 10.1042/BJ20070993. Biochem J. 2008. PMID: 17937660
-
Chaperone-like activity of alpha-crystallin and other small heat shock proteins.Curr Protein Pept Sci. 2001 Sep;2(3):205-25. doi: 10.2174/1389203013381107. Curr Protein Pept Sci. 2001. PMID: 12369933 Review.
-
Structural perturbation of alpha-crystallin and its chaperone-like activity.Int J Biol Macromol. 1998 May-Jun;22(3-4):271-81. doi: 10.1016/s0141-8130(98)00025-7. Int J Biol Macromol. 1998. PMID: 9650082 Review.
Cited by
-
An examination of alpha B-crystallin as a modifier of SOD1 aggregate pathology and toxicity in models of familial amyotrophic lateral sclerosis.J Neurochem. 2010 Jun;113(5):1092-100. doi: 10.1111/j.1471-4159.2010.06572.x. Epub 2010 Jan 7. J Neurochem. 2010. PMID: 20067574 Free PMC article.
-
C-terminal hydrophobic interactions play a critical role in oligomeric assembly of the P22 tailspike trimer.Protein Sci. 2003 Dec;12(12):2732-47. doi: 10.1110/ps.03150303. Protein Sci. 2003. PMID: 14627734 Free PMC article.
-
Unfolding studies of Escherichia coli maltodextrin glucosidase monitored by fluorescence spectroscopy.J Biol Phys. 2008 Dec;34(6):539-50. doi: 10.1007/s10867-008-9117-9. Epub 2008 Nov 12. J Biol Phys. 2008. PMID: 19669512 Free PMC article.
References
-
- Bhosale, S., Rao, M., Deshpande, V., and Srinivasan, M. 1995. Thermostability of high-activity alkaline protease from Conidiobolus coronatus (NCL 86.8.20). Enzyme Microb. Technol. 17 136–139.
-
- Bradford, M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72 248–254. - PubMed
-
- Chiplonkar, J., Gangodkar, S., Wagh, U., Ghadge, G., Rele, M., and Srinivasan, M. 1985. Applications of alkaline protease from Conidiobolus in animal cell culture. Biotechnol. Lett. 7 665–668.
-
- Churchich, J. 1993. Fluorescence properties of o-aminobenzoyl-labeled proteins. Anal. Biochem. 213 229–233. - PubMed
-
- Clark, J. and Muchowski, P. 2000. Small heat-shock proteins and their potential role in human disease. Curr. Opin. Struct. Biol. 10 13–15. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

