Probing metal ion binding and conformational properties of the colicin E9 endonuclease by electrospray ionization time-of-flight mass spectrometry
- PMID: 12070327
- PMCID: PMC2373645
- DOI: 10.1110/ps.0200502
Probing metal ion binding and conformational properties of the colicin E9 endonuclease by electrospray ionization time-of-flight mass spectrometry
Abstract
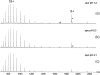
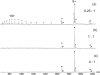
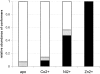
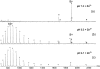
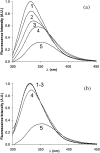
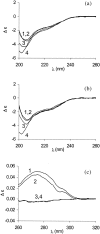
Nano-electrospray ionization time-of-flight mass spectrometry (ESI-MS) was used to study the conformational consequences of metal ion binding to the colicin E9 endonuclease (E9 DNase) by taking advantage of the unique capability of ESI-MS to allow simultaneous assessment of conformational heterogeneity and metal ion binding. Alterations of charge state distributions on metal ion binding/release were correlated with spectral changes observed in far- and near-UV circular dichroism (CD) and intrinsic tryptophan fluorescence. In addition, hydrogen/deuterium (H/D) exchange experiments were used to probe structural integrity. The present study shows that ESI-MS is sensitive to changes of the thermodynamic stability of E9 DNase as a result of metal ion binding/release in a manner consistent with that deduced from proteolysis and calorimetric experiments. Interestingly, acid-induced release of the metal ion from the E9 DNase causes dramatic conformational instability associated with a loss of fixed tertiary structure, but secondary structure is retained. Furthermore, ESI-MS enabled the direct observation of the noncovalent protein complex of E9 DNase bound to its cognate immunity protein Im9 in the presence and absence of Zn(2+). Gas-phase dissociation experiments of the deuterium-labeled binary and ternary complexes revealed that metal ion binding, not Im9, results in a dramatic exchange protection of E9 DNase in the complex. In addition, our metal ion binding studies and gas-phase dissociation experiments of the ternary E9 DNase-Zn(2+)-Im9 complex have provided further evidence that electrostatic interactions govern the gas phase ion stability.
Figures



Similar articles
-
Ligand-induced changes in the conformational dynamics of a bacterial cytotoxic endonuclease.Biochemistry. 2004 Apr 13;43(14):4347-55. doi: 10.1021/bi049929c. Biochemistry. 2004. PMID: 15065879
-
Multistep binding of transition metals to the H-N-H endonuclease toxin colicin E9.Biochemistry. 2002 Aug 13;41(32):10234-44. doi: 10.1021/bi020174o. Biochemistry. 2002. PMID: 12162738
-
Specificity in protein-protein recognition: conserved Im9 residues are the major determinants of stability in the colicin E9 DNase-Im9 complex.Biochemistry. 1998 Jan 13;37(2):476-85. doi: 10.1021/bi971884a. Biochemistry. 1998. PMID: 9425068
-
Electrospray ionization mass spectrometry of oligonucleotide complexes with drugs, metals, and proteins.Mass Spectrom Rev. 2001 Mar-Apr;20(2):61-87. doi: 10.1002/mas.1003. Mass Spectrom Rev. 2001. PMID: 11455562 Review.
-
Ligand-metal ion binding to proteins: investigation by ESI mass spectrometry.Methods Enzymol. 2005;402:361-89. doi: 10.1016/S0076-6879(05)02011-2. Methods Enzymol. 2005. PMID: 16401515 Review.
Cited by
-
Using amino acids for probing structural information of cytochrome c by electrospray ionization mass spectrometry.J Am Soc Mass Spectrom. 2004 Nov;15(11):1612-5. doi: 10.1016/j.jasms.2004.07.017. J Am Soc Mass Spectrom. 2004. PMID: 15519228
-
Advanced mass spectrometry-based methods for the analysis of conformational integrity of biopharmaceutical products.Curr Pharm Biotechnol. 2011 Oct;12(10):1517-29. doi: 10.2174/138920111798357311. Curr Pharm Biotechnol. 2011. PMID: 21542797 Free PMC article. Review.
-
Electrospray ionization mass spectrometry: a technique to access the information beyond the molecular weight of the analyte.Int J Anal Chem. 2012;2012:282574. doi: 10.1155/2012/282574. Epub 2011 Dec 15. Int J Anal Chem. 2012. PMID: 22611397 Free PMC article.
-
Desalting protein ions in native mass spectrometry using supercharging reagents.Analyst. 2014 Oct 7;139(19):4810-9. doi: 10.1039/c4an01085j. Analyst. 2014. PMID: 25133273 Free PMC article.
-
Stabilization of protein structure through π-π interaction in the second coordination sphere of pseudoazurin.Protein Sci. 2017 Oct;26(10):1921-1931. doi: 10.1002/pro.3226. Epub 2017 Jul 20. Protein Sci. 2017. PMID: 28691165 Free PMC article.
References
-
- Bychkova, V.E., Dujsekina, A.E., Klenin, S.I., Tiktopulo, E.I., Uversky, V.N., and Ptitsyn, O.B. 1996. Molten globule-like state of cytochrome c under conditions simulating those near the membrane surface. Biochemistry 35 6058–6063. - PubMed
-
- Cech, N.B. and Enke, C.G. 2000. Relating electrospray ionization response to nonpolar character of small peptides. Anal. Chem. 72 2717–2723. - PubMed
-
- ———. 2001. Effect of affinity for droplet surfaces on the fraction of analyte molecules charged during electrospray droplet fission. Anal. Chem. 73 4632–4639. - PubMed
-
- Chak, K.F., Kuo, W.S., Lu, F.M., and James, R. 1991. Cloning and characterization of the ColE7 plasmid. J. Gen. Microbiol. 137 91–100. - PubMed
-
- Chak, K.-F., Hsieh, S.-Y., Lao, C.-C., and Kan, L.-S. 1998. Change of thermal stability of colicin E7 triggered by acidic pH suggests the existence of unfolded intermediate during the membrane-translocation phase. Proteins 32 17–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

