Structural basis for cyclodextrins' suppression of human growth hormone aggregation
- PMID: 12070330
- PMCID: PMC2373647
- DOI: 10.1110/ps.0202702
Structural basis for cyclodextrins' suppression of human growth hormone aggregation
Abstract
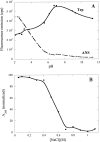
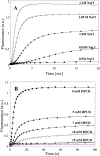
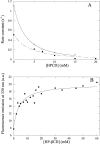
Many therapeutic proteins require storage at room temperature for extended periods of time. This can lead to aggregation and loss of function. Cyclodextrins (CDs) have been shown to function as aggregation suppressors for a wide range of proteins. Their potency is often ascribed to their affinity for aromatic amino acids, whose surface exposure would otherwise lead to protein association. However, no detailed structural studies are available. Here we investigate the interactions between human growth hormone (hGH) and different CDs at low pH. Although hGH aggregates readily at pH 2.5 in 1 M NaCl to form amorphous aggregates, the presence of 25 to 50 mM of various beta-CD derivatives is sufficient to completely avoid this. alpha- and gamma-CD are considerably less effective. Stopped-flow data on the aggregation reaction in the presence of beta-CD are analyzed according to a minimalist association model to yield an apparent hGH-beta-CD dissociation constant of approximately 6 mM. This value is very similar to that obtained by simple fluorescence-based titration of hGH with beta-CD. Nuclear magnetic resonance studies indicate that beta-CD leads to a more unfolded conformation of hGH at low pH and predominantly binds to the aromatic side-chains. This indicates that aromatic amino acids are important components of regions of residual structure that may form nuclei for aggregation.
Figures





References
-
- Abildgaard, F., Jorgensen, A.M.M., Led, J.J., Christensen, T., Jensen, E.B., Junker, F., and Dalboge, H. 1992. Characterization of tertiary interactions in a folded protein by NMR methods: Studies of pH-induced structural changes in human growth hormone. Biochemistry 31 8587–8596. - PubMed
-
- Banga, A.K. and Mitra, R. 1993. Minimization of shaking-induced formation of insoluble aggregates of insulin by cyclodextrins. J. Drug. Target. 1 341–345. - PubMed
-
- Bartels, C., Xia, T.-H., Billeter, M., Günter, P., and Wüthrich, K. 1995. The program XEASY for computer-supported NMR spectral of biological macromolecules. J. Biomol. NMR 5 1–10. - PubMed
-
- Branchu, S., Forbes, R.T., York, P., Petrén, S., Nyqvist, H., and Camber, O. 1999. Hydroxypropyl-β-cyclodextrin inhibits spray-drying–induced inactivation of β-galactosidase. J. Pharm. Sci. 88 905–911. - PubMed
-
- Brems, D.N. 1988. Solubility of different conformers of bovine growth hormone. Biochemistry 27 4541–4546.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

