Design of protein struts for self-assembling nanoconstructs
- PMID: 12070352
- PMCID: PMC124284
- DOI: 10.1073/pnas.132544299
Design of protein struts for self-assembling nanoconstructs
Abstract
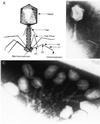
Bacteriophage T4 tail fibers have a quaternary structure of bent rigid rods, 3 x 160 nm in size. The four proteins which make up these organelles are able to self-assemble in an essentially irreversible manner. To use the self-assembly domains of these proteins as elements in construction of mesoscale structures, we must be able to rearrange these domains without affecting the self-assembly properties and add internal binding sites for other functional elements. Here we present results on several alterations of the P37 component of the T4 tail fiber that change its length and add novel protein sequences into the protein. One of these sequences is an antibody binding site that is used to inactivate phage carrying the modified gene.
Figures

Similar articles
-
Evolution of bacteriophage tails: Structure of T4 gene product 10.J Mol Biol. 2006 May 5;358(3):912-21. doi: 10.1016/j.jmb.2006.02.058. Epub 2006 Mar 9. J Mol Biol. 2006. PMID: 16554069
-
Stoichiometry and domainal organization of the long tail-fiber of bacteriophage T4: a hinged viral adhesin.J Mol Biol. 1996 Aug 2;260(5):767-80. doi: 10.1006/jmbi.1996.0436. J Mol Biol. 1996. PMID: 8709154
-
Structure of bacteriophage T4 gene product 11, the interface between the baseplate and short tail fibers.J Mol Biol. 2000 Aug 25;301(4):975-85. doi: 10.1006/jmbi.2000.3989. J Mol Biol. 2000. PMID: 10966799
-
The bacteriophage T4 DNA injection machine.Curr Opin Struct Biol. 2004 Apr;14(2):171-80. doi: 10.1016/j.sbi.2004.02.001. Curr Opin Struct Biol. 2004. PMID: 15093831 Review.
-
Bacteriophage T4: structure, assembly, and initiation infection studied in three dimensions.Adv Virus Res. 2004;63:287-352. doi: 10.1016/S0065-3527(04)63005-3. Adv Virus Res. 2004. PMID: 15530564 Review. No abstract available.
Cited by
-
The Robust Self-Assembling Tubular Nanostructures Formed by gp053 from Phage vB_EcoM_FV3.Viruses. 2019 Jan 11;11(1):50. doi: 10.3390/v11010050. Viruses. 2019. PMID: 30641882 Free PMC article.
-
Functionalized Protein Nanotubes Based on the Bacteriophage vB_KleM-RaK2 Tail Sheath Protein.Nanomaterials (Basel). 2021 Nov 12;11(11):3031. doi: 10.3390/nano11113031. Nanomaterials (Basel). 2021. PMID: 34835795 Free PMC article.
-
Self-Assembly of Tail Tube Protein of Bacteriophage vB_EcoS_NBD2 into Extremely Long Polytubes in E. coli and S. cerevisiae.Viruses. 2019 Mar 1;11(3):208. doi: 10.3390/v11030208. Viruses. 2019. PMID: 30832262 Free PMC article.
-
RNA nanotechnology: engineering, assembly and applications in detection, gene delivery and therapy.J Nanosci Nanotechnol. 2005 Dec;5(12):1964-82. doi: 10.1166/jnn.2005.446. J Nanosci Nanotechnol. 2005. PMID: 16430131 Free PMC article. Review.
-
Exploring the atomic structure and conformational flexibility of a 320 Å long engineered viral fiber using X-ray crystallography.Acta Crystallogr D Biol Crystallogr. 2014 Feb;70(Pt 2):342-53. doi: 10.1107/S1399004713027685. Epub 2014 Jan 29. Acta Crystallogr D Biol Crystallogr. 2014. PMID: 24531468 Free PMC article.
References
-
- Wood W B, Eiserling F A, Crowther R A. In: Molecular Biology of Bacteriophage T4. Karam J D, editor. Washington, DC: Am. Soc. Microbiol. Press; 1994. pp. 282–290.
-
- Henning U, Hashemolhosseini S. In: Molecular Biology of Bacteriophage T4. Karam J D, editor. Washington, DC: Am. Soc. Microbiol. Press; 1994. pp. 291–298.
-
- Wood W B. Harvey Lect. 1979;73:203–223. - PubMed
-
- Eiserling F A, Black L W. In: Molecular Biology of Bacteriophage T4. Karam J D, editor. Washington, DC: Am. Soc. Microbiol. Press; 1994. pp. 209–212.
-
- Beckendorf S K. J Mol Biol. 1973;73:37–53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

