Neutralizing anti-F glycoprotein and anti-substance P antibody treatment effectively reduces infection and inflammation associated with respiratory syncytial virus infection
- PMID: 12072488
- PMCID: PMC136305
- DOI: 10.1128/jvi.76.14.6873-6881.2002
Neutralizing anti-F glycoprotein and anti-substance P antibody treatment effectively reduces infection and inflammation associated with respiratory syncytial virus infection
Abstract
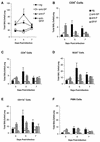
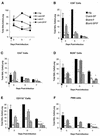
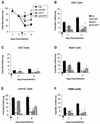
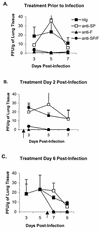
Respiratory syncytial virus (RSV) is the most important virus mediating lower respiratory tract illness in infants and young children. RSV infection is associated with pulmonary inflammation and increased levels of substance P (SP), making the airways and leukocytes that express SP receptors susceptible to the proinflammatory effects of this peptide. This study examines combining neutralizing anti-F glycoprotein and anti-SP antibody treatment of RSV-infected BALB/c mice to inhibit RSV replication and inflammation associated with infection. BALB/c mice were prophylactically treated with antibody prior to RSV infection or were therapeutically treated at day 2 or 6 post-RSV infection. Prophylactic or therapeutic treatment with anti-SP antibodies promptly reduced pulmonary inflammatory cell infiltration and decreased the number of cells expressing proinflammatory cytokines, while anti-F antibody treatment reduced virus titers. The results suggest that combined anti-viral and anti-SP antibody treatment may be effective in treating RSV disease.
Figures
Similar articles
-
Discovery of a Prefusion Respiratory Syncytial Virus F-Specific Monoclonal Antibody That Provides Greater In Vivo Protection than the Murine Precursor of Palivizumab.J Virol. 2017 Jul 12;91(15):e00176-17. doi: 10.1128/JVI.00176-17. Print 2017 Aug 1. J Virol. 2017. PMID: 28539438 Free PMC article.
-
Immunoprophylaxis and immunotherapy of respiratory syncytial virus-infected mice with respiratory syncytial virus-specific immune serum.Pediatr Res. 1993 Aug;34(2):167-72. doi: 10.1203/00006450-199308000-00013. Pediatr Res. 1993. PMID: 8233720
-
Effects of anti-g and anti-f antibodies on airway function after respiratory syncytial virus infection.Am J Respir Cell Mol Biol. 2014 Jul;51(1):143-54. doi: 10.1165/rcmb.2013-0360OC. Am J Respir Cell Mol Biol. 2014. PMID: 24521403 Free PMC article.
-
Development and clinical applications of novel antibodies for prevention and treatment of respiratory syncytial virus infection.Vaccine. 2017 Jan 11;35(3):496-502. doi: 10.1016/j.vaccine.2016.09.026. Epub 2016 Sep 28. Vaccine. 2017. PMID: 27692523 Free PMC article. Review.
-
Prevention of Respiratory Syncytial Virus Infection: From Vaccine to Antibody.Microbiol Spectr. 2014 Aug;2(4):AID-0014-2014. doi: 10.1128/microbiolspec.AID-0014-2014. Microbiol Spectr. 2014. PMID: 26104207 Review.
Cited by
-
Preparation and Evaluation of the Fully Humanized Monoclonal Antibody GD-mAb Against Respiratory Syncytial Virus.Front Cell Infect Microbiol. 2019 Jul 31;9:275. doi: 10.3389/fcimb.2019.00275. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31417879 Free PMC article.
-
Induction of tachykinin production in airway epithelia in response to viral infection.PLoS One. 2008 Mar 5;3(3):e1673. doi: 10.1371/journal.pone.0001673. PLoS One. 2008. PMID: 18320026 Free PMC article.
-
Anti-respiratory syncytial virus (RSV) G monoclonal antibodies reduce lung inflammation and viral lung titers when delivered therapeutically in a BALB/c mouse model.Antiviral Res. 2018 Jun;154:149-157. doi: 10.1016/j.antiviral.2018.04.014. Epub 2018 Apr 17. Antiviral Res. 2018. PMID: 29678551 Free PMC article.
-
Anti-respiratory syncytial virus (RSV) neutralizing antibody decreases lung inflammation, airway obstruction, and airway hyperresponsiveness in a murine RSV model.Antimicrob Agents Chemother. 2004 May;48(5):1811-22. doi: 10.1128/AAC.48.5.1811-1822.2004. Antimicrob Agents Chemother. 2004. PMID: 15105140 Free PMC article.
-
Vasoactive Peptides: Role in COVID-19 Pathogenesis and Potential Use as Biomarkers and Therapeutic Targets.Arch Med Res. 2021 Nov;52(8):777-787. doi: 10.1016/j.arcmed.2021.05.007. Epub 2021 Jun 5. Arch Med Res. 2021. PMID: 34134920 Free PMC article. Review.
References
-
- Berczi, I., I. M. Chalmers, E. Nagy, and R. J. Warrington. 1996. The immune effects of neuropeptides. Baillieres Clin. Rheumatol. 10:227-257. - PubMed
-
- Berman, A. S., C. Chancellor-Freeland, G. Zhu, and P. H. Black. 1996. Substance P primes murine peritoneal macrophages for an augmented proinflammatory cytokine response to lipopolysaccharide. Neuroimmunomodulation 3:141-149. - PubMed
-
- Calvo, N., J. Reiriz, E. Perez-Navarro, and J. Alberch. 1996. Tachykinins protect cholinergic neurons from quinolinic acid excitotoxicity in striatal cultures. Brain Res. 740:323-328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

