AIDS vaccination studies using an ex vivo feline immunodeficiency virus model: failure to protect and possible enhancement of challenge infection by four cell-based vaccines prepared with autologous lymphoblasts
- PMID: 12072489
- PMCID: PMC136316
- DOI: 10.1128/jvi.76.14.6882-6892.2002
AIDS vaccination studies using an ex vivo feline immunodeficiency virus model: failure to protect and possible enhancement of challenge infection by four cell-based vaccines prepared with autologous lymphoblasts
Abstract
Immunogenicity and protective activity of four cell-based feline immunodeficiency virus (FIV) vaccines prepared with autologous lymphoblasts were investigated. One vaccine was composed of FIV-infected cells that were paraformaldehyde fixed at the peak of viral expression. The other vaccines were attempts to maximize the expression of protective epitopes that might become exposed as a result of virion binding to cells and essentially consisted of cells mildly fixed after saturation of their surface with adsorbed, internally inactivated FIV particles. The levels of FIV-specific lymphoproliferation exhibited by the vaccinees were comparable to the ones previously observed in vaccine-protected cats, but antibodies were largely directed to cell-derived constituents rather than to truly viral epitopes and had very poor FIV-neutralizing activity. Moreover, under one condition of testing, some vaccine sera enhanced FIV replication in vitro. As a further limit, the vaccines proved inefficient at priming animals for anamnestic immune responses. Two months after completion of primary immunization, the animals were challenged with a low dose of homologous ex vivo FIV. Collectively, 8 of 20 vaccinees developed infection versus one of nine animals mock immunized with fixed uninfected autologous lymphoblasts. After a boosting and rechallenge with a higher virus dose, all remaining animals became infected, thus confirming their lack of protection.
Figures
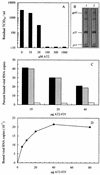
 ), or AT2-inactivated and then disrupted by sonication (□). After 2 h at 4°C, the PLB were pelleted and examined for bound FIV RNA copies by quantitative TM-PCR. The results are expressed as the percent viral RNA copies found associated with the cells relative to the number of input copies. (D) Dose curve of AT2-FIV binding to PLB. Indicated amounts of AT2-FIV were incubated with 5 × 105 PLB which, after 2 h at 4°C, were pelleted and examined as described above for bound viral RNA. The results are expressed as the numbers of viral RNA copies found associated with the cells.
), or AT2-inactivated and then disrupted by sonication (□). After 2 h at 4°C, the PLB were pelleted and examined for bound FIV RNA copies by quantitative TM-PCR. The results are expressed as the percent viral RNA copies found associated with the cells relative to the number of input copies. (D) Dose curve of AT2-FIV binding to PLB. Indicated amounts of AT2-FIV were incubated with 5 × 105 PLB which, after 2 h at 4°C, were pelleted and examined as described above for bound viral RNA. The results are expressed as the numbers of viral RNA copies found associated with the cells.

References
-
- Arthur, L. O., J. W. Bess, Jr., E. N. Chertova, J. L. Rossio, M. T. Esser, R. E. Benveniste, L. E. Henderson, and J. D. Lifson. 1998. Chemical inactivation of retroviral infectivity by targeting nucleocapsid protein zinc fingers: a candidate SIV vaccine. AIDS Res. Hum. Retrovir. 14:S311-S319. - PubMed
-
- Binley, J. M., R. W. Sanders, B. Clas, N. Schuelke, A. Master, Y. Guo, F. Kajumo, D. J. Anselma, P. J. Maddon, W. C. Olson, and J. P. Moore. 2000. A recombinant human immunodeficiency virus type 1 envelope glycoprotein complex stabilized by an intermolecular disulfide bond between the gp120 and gp41 subunits is an antigenic mimic of the trimeric virion-associated structure. J. Virol. 74:627-643. - PMC - PubMed
-
- Bishop, S. A., C. R. Stokes, T. J. Gruffydd-Jones, C. V. Whiting, J. E. Humphries, R. Osborne, M. Papanastasopoulou, and D. A. Harbour. 1996. Vaccination with fixed feline immunodeficiency virus (FIV)-infected cells: protection, breakthrough, and specificity of response. Vaccine 14:1243-1250. - PubMed
-
- Bogers, W. M. J. M., C. Cheng-Mayer, and R. C. Montelaro. 2000. Developments in preclinical AIDS vaccine efficacy models. AIDS 14:S141-S151. - PubMed
-
- Burton, D. R., and P. W. H. I. Parren. 2000. Vaccines and the induction of functional antibodies: time to look beyond the molecules of natural infection? Nat. Med. 6:123-125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

