The amino-terminal half of Sendai virus C protein is not responsible for either counteracting the antiviral action of interferons or down-regulating viral RNA synthesis
- PMID: 12072511
- PMCID: PMC136303
- DOI: 10.1128/jvi.76.14.7114-7124.2002
The amino-terminal half of Sendai virus C protein is not responsible for either counteracting the antiviral action of interferons or down-regulating viral RNA synthesis
Abstract
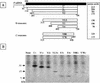
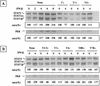
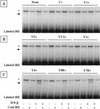
The Sendai virus C proteins, C', C, Y1, and Y2, are a nested set of independently initiated carboxy-coterminal proteins translated from a reading frame overlapping the P frame on the P mRNA. The C proteins are extremely versatile and have been shown to counteract the antiviral action of interferons (IFNs), to down-regulate viral RNA synthesis, and to promote virus assembly. Using the stable cell lines expressing the C, Y1, Y2, or truncated C protein, we investigated the region responsible for anti-IFN action and for down-regulating viral RNA synthesis. Truncation from the amino terminus to the middle of the C protein maintained the inhibition of the signal transduction of IFNs, the formation of IFN-stimulated gene factor 3 (ISGF3) complex, the generation of the anti-vesicular stomatitis virus state, and the synthesis of viral RNA, but further truncation resulted in the simultaneous loss of all of these inhibitory activities. A relatively small truncation from the carboxy terminus also abolished all of these inhibitory activities. These data indicated that the activities of the C protein to counteract the antiviral action of IFNs and to down-regulate viral RNA synthesis were not encoded within a region of at least 98 amino acids in its amino-terminal half.
Figures

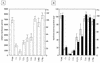
References
-
- Curran, J., J.-B. Marq, and D. Kolakofsky. 1992. The Sendai virus nonstructural C proteins specifically inhibit viral mRNA synthesis. Virology 189:647-656. - PubMed
-
- Delenda, C., S. Hausmann, D. Garcin, and D. Kolakofsky. 1997. Normal cellular replication of Sendai virus without the trans-frame, nonstructural V protein. Virology 228:55-62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

