Characterization of varicella-zoster virus gene 21 and 29 proteins in infected cells
- PMID: 12072522
- PMCID: PMC136324
- DOI: 10.1128/jvi.76.14.7228-7238.2002
Characterization of varicella-zoster virus gene 21 and 29 proteins in infected cells
Abstract
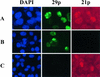
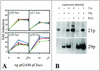
Varicella-zoster virus (VZV) transcription is limited in latently infected human ganglia. Note that much of the transcriptional capacity of the virus genome has not been analyzed in detail; to date, only VZV genes mapping to open reading frames (ORFs) 4, 21, 29, 62, and 63 have been detected. ORF 62 encodes the major immediate-early virus transcription transactivator IE62, ORF 29 encodes the major virus DNA binding protein, and ORF 21 encodes a protein associated with the developing virus nucleocapsid. We analyzed the cellular location of proteins encoded by ORF 21 (21p) and ORF 29 (29p), their phosphorylation state during productive infection, and their ability form a protein-protein complex. The locations of both 21p and 29p within infected cells mimic those of their herpes simplex virus type 1 (HSV-1) homologues (UL37 and ICP8); however, unlike these homologues, 21p is not phosphorylated and neither 21p nor 29p exhibits a protein-protein interaction. Transient transfection assays to determine the effect of 21p and 29p on transcription from VZV gene 20, 21, 28, and 29 promoters revealed no significant activation of transcription by 21p or 29p from any of the VZV gene promoters tested, and 21p did not significantly modulate the ability of IE62 to activate gene transcription. A modest increase in IE62-induced activation of gene 28 and 29 promoters was seen in the presence of 29p; however, IE62-induced activation of gene 28 and 29 promoters was reduced in the presence of 21p. A Saccharomyces cerevisiae two-hybrid analysis of 21p indicated that the protein can activate transcription when tethered within a responsive promoter. Together, the data reveal that while VZV gene 21 and HSV-1 UL37 share homology at the nucleic acid level, these proteins differ functionally.
Figures





References
-
- Abendroth, A., and A. Arvin. 1999. Varicella-zoster virus immune evasion. Immunol. Rev. 168:143-156. - PubMed
-
- Annunziato, P. W., O. Lungu, and C. Panagiotidis. 2001. Varicella zoster virus in human and rat tissue specimens. Arch. Virol. Suppl. 17:135-142. - PubMed
-
- Ben Ze'ev, A., R. Abulafia, and S. Bratosin. 1983. Herpes simplex virus and protein transport are associated with the cytoskeletal framework and the nuclear matrix in infected BSC-1 cells. Virology 129:501-507. - PubMed
-
- Blom, N., S. Gammeltoft, and S. Brunak. 1999. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294:1351-1362. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

