Characterization of partially purified 8 kDa antigenic protein of Clonorchis sinensis
- PMID: 12073733
- PMCID: PMC2721047
- DOI: 10.3347/kjp.2002.40.2.83
Characterization of partially purified 8 kDa antigenic protein of Clonorchis sinensis
Abstract
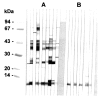
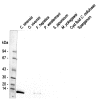
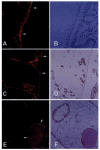
The 8 kDa antigenic protein of Clonorchis sinensis was partially purified by ammonium sulfate precipitation and subsequently by a column chromatographic steps. The purified protein was separated into 7 and 8 kDa protein bands through SDS-tricine gel electrophoresis, while the protein was found to migrate to a 8 kDa band in 7.5-15% SDS-PAGE. The molecular weight of the antigen was estimated to be 110 kDa by Superose 6 HR 10/30 gel filtration. The purified antigen strongly reacted with the human sera of clonorchiasis. The hyperimmune sera of BALB/c mice immunized against the 8 kDa protein were reacted with both the crude extract and the excretory-secretory product of adult worms, but not with the metacercarial extract. Immunohistochemical staining demonstrated that the protein was distributed to the tegument and subtegumental cells and also to the seminal receptacle. The present findings suggest that the 8 kDa protein is a partition of the multicomplex protein originating from various organs of adult C. sinensis, and that it is composed of several 7 and 8 kDa proteins.
Figures


References
-
- Hong ST, Kho WG, Lee M, Lee JS, Lee SH. Immunoblot pattern of clonorchiasis. Korean J Parasitol. 1997;35:87–93. - PubMed
-
- Kang SY, Ann IY, Park CY, et al. Clonorchis sinensis: molecular cloning and characterization of 28-kDa glutathione S-transferase. Exp Parasitol. 2001;97:186–195. - PubMed
-
- Kim SI. Immune reactions between excretory-secretory antigens and specific antibodies of Clonorchis sinensis before and after praziquantel treatment in experimentally. Korean J Parasitol. 1994;32:35–42. - PubMed
-
- Kim SI. A Clonorchis sinensis-specific antigen that detects active human clonorchiasis. Korean J Parasitol. 1998;36:37–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

