Amisulpride for schizophrenia
- PMID: 12076408
- PMCID: PMC7045777
- DOI: 10.1002/14651858.CD001357
Amisulpride for schizophrenia
Abstract
Background: The treatment of schizophrenia with old, 'typical' antipsychotic drugs such as haloperidol can be problematic, because many people treated with these drugs will suffer from movement disorders. Amisulpride is said to be an "atypical" antipsychotic which induces less movement disorder and which is effective for the negative symptoms of schizophrenia.
Objectives: To evaluate the effects of amisulpride as compared with placebo, typical and atypical antipsychotic drugs for schizophrenia.
Search strategy: The authors carried out electronic searches of Biological Abstracts (1982-1999), CINAHL (1982-1999), Cochrane Library (Issue 4, 1999), Cochrane Schizophrenia Group's Register (November 2000), EMBASE (1980-1999), LILACS(1982-1999), MEDLINE (1966-1999) and PsycLIT (1974-1999). They checked all identified studies for further trial citations, and sought these studies in the Science Citation Index. They also contacted authors of trials and the manufacturer of amisulpride.
Selection criteria: All randomised controlled trials comparing amisulpride to placebo, typical or atypical antipsychotic drugs for schizophrenia or other non-affective serious mental illnesses.
Data collection and analysis: Data were independently extracted and analysed on an intention-to-treat basis. The relative risk (RR) and 95% confidence intervals (CI) of dichotomous data were calculated using a random effects model, and, where possible, the number needed to treat was calculated. Weighted mean differences (WMD) were calculated for continuous data.
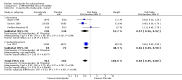
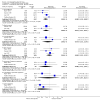
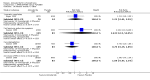
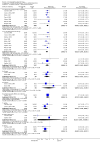
Main results: This review currently includes 19 randomised studies with a total of 2443 participants. Most trials were of short duration. Data from 4 trials with 514 participants with predominantly negative symptoms suggest that low-dose (up to 300mg/day) amisulpride was a more acceptable treatment than placebo (n=514, RR 0.6 CI 0.5 to 0.8, NNT 3 CI 3 to 7), the improvement of the participants' global state (n=242, RR 0.6 CI 0.5 to 0.8, NNT 3 CI 2 to 6) and the treatment of negative symptoms (n=177, WMD -10.1 CI -16.6 to -3.5). Amisulpride was shown to be more likely to cause extrapyramidal symptoms than placebo in two studies (n=269, RR 2.2 CI 1.2 to 4.2), but this result did not hold calculating the risk reduction so that an NNT-statistic could not be indicated. Compared to typical antipsychotics, the pooled results of a total of fourteen trials suggest that amisulpride was more effective in improving global state (n=651, RR 0.7 CI 0.5 to 0.9, NNT 6 CI 4 to 11), the general mental state (n=695, WMD -4.2 CI -6.5 to -1.9) and the negative symptoms of schizophrenia (n=506, WMD -2.8 CI -4.3 to -1.3). Regarding positive symptoms, amisulpride was as effective as typical antipsychotics. Amisulpride was less prone to cause at least one general adverse event (n=751, RR 0.9 CI 0.8 to 0.97, NNH 9 CI 6 to 18), one extrapyramidal symptom (n=771, RR 0.7 CI 0.6 to 0.9, NNH 5 CI 4 to 9) or to require the use of antiparkinson medication (n=851, RR 0.6 CI 0.5 to 0.8, NNH 4 CI 3 to 6). No clear differences in other adverse events compared to typical drugs were found. Amisulpride also seemed to be more acceptable than conventional drugs as measured by the outcome 'leaving the studies early' (n=1512, RR 0.8 CI 0.7 to 0.9, NNT 16 CI 9 to 69) than conventional drugs, but this result might have been overestimated due to a publication bias which could not be excluded with certainty. A single trial compared amisulpride to another 'atypical' antipsychotic, risperidone. With the exception of agitation, which was more frequent in the amisulpride group (n=228, RR 3.4 CI 1.2 to 10.1, NNH 11 CI 6 to 50) no significant differences were recorded on efficacy or acceptability.
Reviewer's conclusions: This systematic review confirms that amisulpride is an effective 'atypical' antipsychotic drug for those with schizophrenia. Amisulpride may offer a good general profile, at least compared to high-potency 'typical' antipsychotics. It may also yield better results in some specific outcomes related to efficacy, such as improvement of global state and general negative symptoms. It might be more acceptable and more tolerable than high-potency conventional antipsychotics, especially regarding extrapyramidal side-effects. Longer term randomised trials are needed to evaluate the comparative value of amisulpride, particularly compared to other expensive atypical antipsychotics. These should focus on important outcomes which have not been sufficiently monitored such as service use, family burden and quality of life.
Conflict of interest statement
None known.
Figures






















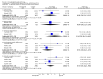
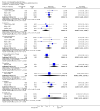
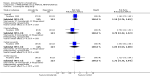





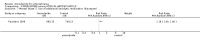
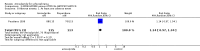



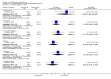









References
References to studies included in this review
Boyer 1995 {published data only}
-
- Boyer P. Amisulpride in the treatment of negative schizophrenic symptoms: results of a double‐blind controlled trial versus placebo. In: Borenstein P editor(s). Amisulpride. Paris: Expansion Scientifique Française, 1989:111‐23.
-
- Boyer P, Lecrubier Y, Puech AJ, Dewailly, Aubin F. Treatment of negative symptoms in schizophrenia with amisulpride. British Journal of Psychiatry 1995;166:68‐72. - PubMed
-
- Boyer P, Lecrubier Y, Puech JA. Treatment of positive and negative symptoms: pharmacologic approaches. In: Andreasen NC editor(s). Schizophrenia: positive and negative symptoms and syndromes. Modern Problems of Pharmacopsychiatry. Vol. 24, Basel: Karger, 1990:152‐74. - PubMed
-
- Boyer P, Puech AJ. Determinants for clinical activity of neuroleptic drugs: chemical substances, doses, assessment tools [Modalités d'action clinique des neuroleptiques: substances, doses, instruments de mesure utilisés]. Psychiatrie and Psychobiologie 1987;4:296‐305.
-
- Boyer P, Puech AJ, Lecrubier Y. Double blind trial versus placebo of low dose amisulpride (Solian 50) in schizophrenia with exclusively negative symptoms. Preliminary analysis of results [Étude en double insu contre placebo de l'amisulpride (Solian 50) à faible dose chez des schizophrènes purement déficitaires. Première analyse des rèsultats]. Annals of Psychiatry 1988;3(3 bis):321‐5.
Carrière 2000 {published data only}
-
- Carrière P, Bonhomme D, Lempérière T. Amisulpride has a superior benefit/risk profile to haloperidol in schizophrenia: results of a multicentre double‐blind study (the Amisulpride Study Group). European Psychiatry 2000;15:321‐9. - PubMed
Colonna 2000 {published data only}
-
- Colonna L, Guerault E, Turjanski S. Long‐term study of amisulpride in the treatment of schizophrenia. Biological Psychiatry 1997;42(Suppl. 1):181S‐2S.
-
- Colonna L, Martin P, Gerard D. [Etude à long terme de l'efficacité et de la tolérance à l'amisulpride versus haloperidol]. Congrés de Psychiatrie et de Neurologie de Langue Française, Saint Paul; 1998. 1998.
-
- Colonna L, Rein W, Turjanski S. Maintenance of antipsychotic efficacy with amisulpride: results of a long‐term study versus haloperidol. Abstracts of the 9th Congress of the Association of European Psychiatrists; 1998 Sept 20‐24; Copenhagen, Denmark. 1998.
-
- Colonna L, Rein W, Turjanski S. Maintenance of the antipsychotic efficacy with amisulpride: results of a long‐term study versus haloperidol. Abstracts of the XXIst C.I.N.P. Congress; 1998 July 12‐16; Glasgow, Scotland, UK. 1998.
-
- Colonna L, Saleem P, Dondey‐Nouvel L, Rein W, Amisulpride Study Group. Long‐term safety and efficacy of amisulpride in sub‐chronic or chronic schizophrenia. International Clinical Psychopharmacology 2000;15(1):13‐22. - PubMed
Costa e Silva 1989 {published data only}
-
- Costa e Silva JA. Comparative double‐blind study of amisulpride versus haloperidol in the treatment of acute psychotic states. In: Borenstein P editor(s). Amisulpride. Paris. Expansion Scientifique Française, 1989:93‐104.
-
- Costa e Silva JA. Comparative double‐blind study of amisulpride versus haloperidol in the treatment of acute psychotic states. Annals of Psychiatry 1990;5(1):71‐8.
Danion 1999 {published data only}
-
- Danion JM, Rein W, Fleurot O, Amisulpride Study Group. Improvement of schizophrenic patients with primary negative symptoms treated with amisulpride. American Journal of Psychiatry 1999;156:610‐6. - PubMed
-
- Danion JM, Turjanski S, Fleurot O, Rein W. Amisulpride in primary negative symptoms of schizophrenia. Abstracts of the 9th Congress of the Association of European Psychiatrists; 1998 Sept 20‐24; Copenhagen, Denmark. 1998.
-
- Danion JM, Turjanski S, Fleurot O, Rein W. Amisulpride in primary negative symptoms of schizophrenia. Abstracts of the XXIst C.I.N.P. Congress; 1998 July 12‐16; Glasgow, Scotland, UK. 1998.
Delcker 1990 {published data only}
-
- Delcker A, Schoon ML, Oczkowski B, Gaertner HJ. Amisulpride versus haloperidol in treatment of schizophrenic patients ‐ results of a double‐blind study. Pharmacopsychiatry 1990;23:125‐30. - PubMed
Klein 1985 {published data only}
-
- Klein HE. A double‐blind comparison of amisulpride versus haloperidol in acute schizophrenics patients. Abstracts WPA Congress; 1983; Vienna, Austria. 1983:687‐90.
-
- Klein HE, Dieterle D, Rüther E, Eben E, Nedopil N, Hippius H. A double‐blind comparison of amisulpride versus haloperidol in acute schizophrenics patients. In: Pichot P, Berner P, Wolf R, Thau K editor(s). Psychiatry, "The State of the Art". Vol. 3, Plenum Press, 1985:687‐91.
-
- Rüther E, Eben E, Klein H, Nedopil N, Dieterle D, Hippius H. Comparative double‐blind study of amisulpride versus haloperidol in the treatment of acute episodes of positive schizophrenia. In: Borenstein P editor(s). Amisulpride. Paris: Expansion Scientifique Française, 1989:63‐72.
Loo 1997 {published data only}
-
- Loo H, Poirier‐Littre MF, Theron M, Rein W, Fleurot O. Amisulpride versus placebo in the medium‐term treatment of the negative symptoms of schizophrenia. British Journal of Psychiatry 1997;170:18‐22. - PubMed
-
- Pélissolo A, Krebs MO, Olié JP. Traitement des symptômes déficitaires de la schizophrénie par l'amisulpride. Revue de la littérature. Encephale 1996;22(3):215‐9. - PubMed
-
- Rein W, Turjanski S. Clinical update on amisulpride in deficit schizophrenia. International Clinical Psychopharmacology 1997;12(Suppl 2):S19‐27. - PubMed
Möller 1997 {published data only}
-
- Boyer P, Turjanski S, Fleurot O. Amisulpride in the treatment of acute schizophrenia: a double‐blind comparison with haloperidol. European Neuropsychopharmacology (Abstracts of the XXth Collegium International Neuro‐psychopharmacologicum Congress; 1996 June 23‐27; Melbourne, Australia) 1996;6(Suppl.3):60‐1. - PubMed
-
- Freeman HL. Amisulpride compared with standard neuroleptics in acute exacerbations of schizophrenia: three efficacy studies. International Clinical Psychopharmacology 1997;12(Suppl. 2):S11‐7. - PubMed
-
- Möller H, Boyer P, Rein W, Eich F. Treatment of schizophrenic patients with acute exacerbations: a double‐blind comparison of amisulpride and haloperidol. Pharmacopsychiatry (Abstracts from the 20th Symposium of AGNP; 1997 Oct 8‐11; Nuremberg, Germany). 1997; Vol. 30, issue 5:199.
-
- Möller H, Boyer P, Turjanski S, Fleurot O and the Amisulpride Study Group. Amisulpride in the treatment of subchronic or chronic schizophrenia with acute exacerbation: a double‐blind comparison with haloperidol. 8th Congress of the Association of European Psychiatrists; 1996; London, UK. 1996.
-
- Möller HJ, Boyer P, Fleurot O, Rein W. Improvement of acute exacerbations of schizophrenia with amisulpride: a comparison with haloperidol. Psychopharmacology 1997;132:396‐401. - PubMed
Paillère‐Martinot 95 {published data only}
-
- Paillère‐Martinot ML, Lecrubier Y, Martinot JL, Aubin F. Improvement of some schizophrenic deficit symptoms with low doses of amisulpride. American Journal of Psychiatry 1995;152:130‐3. - PubMed
-
- Pélissolo A, Krebs MO, Olié JP. [Traitement des symptômes déficitaires de la schizophrénie par l'amisulpride. Revue de la littérature]. Encephale 1996;22(3):215‐9. - PubMed
-
- Rein W, Turjanski S. Clinical update on amisulpride in deficit schizophrenia. International Clinical Psychopharmacology 1997;12(Suppl. 2):S19‐27. - PubMed
Peuskens 1999 {published data only}
-
- Fleurot O, Bech P, Turjanski S. Amisulpride versus risperidone in the treatment of acute schizophrenia.. Biological Psychiatry (Abstracts of the 6th Congress of Biological Psychiatry; 1997 June 22‐27; Nice, France). 1997; Vol. 42, issue Suppl. 1:194S.
-
- Peuskens J, Bech P, Möller HJ, Bale R, Fleurot O, Rein W and the Amisulpride Study Group. Amisulpride versus risperidone in the treatment of acute exacerbations of schizophrenia. Psychiatry Research 1999;88(2):107‐17. - PubMed
Pichot 1988a {published data only}
-
- Boyer P. [Étude de l'efficacité de faibles doses de neuroleptiques atypiques (benzamides) dans les états déficitaires]. Annales Mèdico‐Psychologiques 1986;144:593‐9. - PubMed
-
- Boyer P, Lecrubier Y, Puech AJ. Treatment of positive and negative symptoms: pharmacologic approaches. In: Andreasen NC editor(s). Schizophrenia: positive and negative symptoms and syndromes. Modern Problems of Pharmacopsychiatry. Vol. 24, Basel: Karger, 1990:152‐74. - PubMed
-
- Boyer P, Puech AJ. Determinants for clinical activity of neuroleptic drugs: chemical substances, doses, assessment tools [Modalités d'action clinique des neuroleptiques: substances, doses, instruments de mesure utilisés]. Psychiatrie and Psychobiologie 1987;4:296‐305.
-
- Pichot P. Clinical effects of a new substituted benzamide (amisulpride) on the negative and positive symptoms of schizophrenia. 15th Congress of CINP; 1986; San Juan, Porto Rico. 1986.
-
- Pichot P, Boyer P. Controlled double‐blind multi‐centre trial of low dose amisulpride versus fluphenazine in the treatment of the negative syndrome of chronic schizophrenia. Annales de Psychiatrie 1988;3,(3 bis):312‐20.
Pichot 1988b {published data only}
-
- Boyer P. [Étude de l'efficacité de faibles doses de neuroleptiques atypiques (benzamides) dans les états déficitaires]. Annales Mèdico‐Psychologiques 1986;144:593‐9. - PubMed
-
- Boyer P, Lecrubier Y, Puech AJ. Treatment of positive and negative symptoms: pharmacologic approaches. In: Andreasen NC editor(s). Schizophrenia: positive and negative symptoms and syndromes. Modern Problems of Pharmacopsychiatry. Vol. 24, Basel: Karger, 1990:152‐74. - PubMed
-
- Boyer P, Puech AJ. Determinants for clinical activity of neuroleptic drugs: chemical substances, doses, assessment tools [Modalités d'action clinique des neuroleptiques: substances, doses, instruments de mesure utilisés]. Psychiatrie and Psychobiologie 1987;4:296‐305.
-
- Pichot P, Boyer P. A controlled double‐blind multi‐centre trial of high dose amisulpride versus haloperidol in acute psychotic states. Annales de Psychiatrie 1988;3(3 bis):326‐32.
Puech 1998 {published data only}
-
- Freeman HL. Amisulpride compared with standard neuroleptics in acute exacerbations of schizophrenia: three efficacy studies. International Clinical Psychopharmacology 1997;12(Suppl. 2):S11‐7. - PubMed
-
- Puech A, Fleurot O, Rein W. Amisulpride in the treatment of acute exacerbations of subchronic or chronic schizophrenia: a dose‐ranging study. European Psychiatry 1996;11(4):280S.
-
- Puech A, Fleurot O, Rein W, Amisulpride Study Group. Amisulpride, an atypical antipsychotic, in the treatment of acute episodes of schizophrenia: a dose‐ranging study versus haloperidol. Acta Psychiatrica Scandinavica 1998;98:65‐72. - PubMed
-
- Puech A, Turjanski S, Fleurot O. Amisulpride in the treatment of acute episodes of schizophrenia. European Neuropsychopharmacology (Abstracts of the XXth Collegium Internationale Neuro‐psychopharmacologicum Congress; 1996 June 23‐27; Melbourne, Australia) 1996;6(Suppl. 3):61. - PubMed
Rüther 1988 {published data only}
-
- Dieterle DM, Albus M, Blanke E, Eben E, Klein H, Müller‐Spahn, Rüther E. A double‐blind comparison of aminosultoprid (DAN 2163) versus perazine in schizophrenic patients. Abstracts of the 4th World Congress of Biological Psychiatry; 1989 Sept 8‐13; Philadelphia, USA. 1989.
-
- Rüther E, Blanke J. [Therapievergleich von Aminosultoprid (DAN 2163) versus Perazin bei schizophrenen Patienten]. In: Helmchen H, Hippius H editor(s). Therapie mit Neuroleptika ‐ Perazin. Stuttgart, New York: Thieme‐Verlag, 1988:65‐70.
Saletu 1994 {published data only}
-
- Saletu B, Küfferle B, Grünberger J, Földes P, Topitz A, Anderer P. Clinical, EEG mapping and psychometric studies in negative schizophrenia: comparative trials with amisulpride and fluphenazine. Neuropsychobiology 1994;29:125‐35. - PubMed
Speller 1997 {published data only}
-
- Barnes TRE, Speller JC, Curson DA, Pantelis C, Alberts JL. A one‐year dose reduction study in chronic schizophrenic inpatients: amisulpride versus haloperidol. Schizophrenia Research 1992;6:107.
-
- Speller JC, Barnes TRE, Curson DA, Pantelis C, Alberts JL. One‐year, low‐dose neuroleptic study of in‐patients with chronic schizophrenia characterised by persistent negative symptoms. Amisulpride versus haloperidol. British Journal of Psychiatry 1997;171:564‐8. - PubMed
Wetzel 1998 {published data only}
-
- Freeman HL. Amisulpride compared with standard neuroleptics in acute exacerbations of schizophrenia: three efficacy studies. International Clinical Psychopharmacology 1997;12(Suppl. 2):S11‐7. - PubMed
-
- Hillert A, Phillipp M, Gattaz W. Amisulpride versus flupenthixol in the treatment of schizophrenia with predominant positive symptomatology: a controlled double‐blind study [abstract]. Neuropsychopharmacology 1994;10(Suppl. 3, part 2):31S.
-
- Müller MJ, Wetzel H, Benkert O. Differential treatment effects of amisulpride versus flupentixol on latent dimensions of depressive and negative symptoms in acute schizophrenia. Schizophrenia Research 1998;29(1,2):157. - PubMed
-
- Müller MJ, Wetzel H, Benkert O. Latent dimentions of depressive and negative symptoms in acute schizophrenia: treatment effects of high dose amisulpride versus flupentixol. European College of Neuropsychopharmacology Congress; 1998; Paris, France. 1998.
-
- Wetzel H, Gründer G, Hillert A, Philipp M, Gattaz WF, Sauer H, Adler G, Schröder J, Rein W, Benkert O and the Amisulpride Study Group. Amisulpride versus flupentixol in schizophrenia with predominantly positive symptomatology ‐ double‐blind study comparing a selective D2‐like antagonist to a mixed D1‐/D2‐like antagonist. Psychopharmacology 1998;137:223‐32. - PubMed
Ziegler 1989 {published data only}
-
- Ziegler B. A double‐blind study of DAN2163 versus haloperidol in psychotic patients. Rapport d'Expertise, 2163 D451 1985.
-
- Ziegler B. Study of the efficacy of a substituted benzamide amisulpride versus haloperidol, in productive schizophrenia. In: Borenstein P editor(s). Amisulpride. Paris: Expansion Scientifique Francaise, 1989:71‐3.
-
- Ziegler B, Jesau R. [Untersuchungen zur Wirksamkeit eines substituierten Benzamids (DAN 2163) auf schizophrene Symptome. Statistische Auswertung einer Doppleblind prufung mit DAN 2163 gegen Haloperidol bei psychotischen Patienten.]. Rapport d'expertise 2163 D541 1985.
References to studies excluded from this review
Baldauf 1988 {published data only}
-
- Baldauf A, Metzger JY, Sichel JP. Solian: evaluation of 10 years' experimentation and clinical practice. Annales Medicales et Psychologiques 1988;146(1‐2):63‐6. - PubMed
Bogetto 1995 {published data only}
-
- Baldauf A, Metzger JY, Sichel JP. Adjunctive flouxetine or amisulpride improves schizophrenic negative symptoms. European Journal of Psychiatry 1995;9(2):119‐26.
Brunner 1987 {published data only}
-
- Brunner H, Croisy JL, Crennar A. [Efficacité thérapeutique et tolérance du Solian dans le traitement de l'inhibition]. Actualités en Psychiatrie 1987;17:90‐7.
Casey 1997 {published data only}
-
- Casey DE. Comprehensive drug treatment of schizophrenia: the impact of a non‐conventional antipsychotic amisulpride. Introduction. International Clinical Psychopharmacology 1997;12(Suppl. 2):S1‐2.
Cassan 1985 {published data only}
-
- Cassan P. Benzamides, a therapeutic response adapted to two sides, paranoid and hebephrenic, of schizophrenia. Annales Medico Psychologiques 1985;143(7):677‐82. - PubMed
Chabannes 1998 {published data only}
-
- Chabannes JP, Pelissolo A, Farah S, Gerard D. Evaluation of the effects and tolerance of amisulpride in the treatment of psychotic schizophrenics [Evaluation de l'efficacité et de la tolérance de l'amisulpride dans le traitement des psychoses schizophréniques]. Encephale 1998;24(4):386‐92. - PubMed
Clerc 1989 {published data only}
-
- Clerc G. Double blind study of DAN 2163 in different dosages with schizophrenia patients [Étude en double aveugle du DAN 2163 à différentes posologies chez les malades schizophrenes]. Rapport d'expertise 2163 D162, 1981.
-
- Clerc G. Double‐blind study of amisulpride at different dosages in negative schizophrenic patients. In: Borenstein P editor(s). Amisulpride. Paris: Expansion Scientifique Française, 1989:105‐10.
-
- Clerc G. Double‐blind study of amisulpride at different dosages in negative schizophrenic patients. Semaine des Hôpitaux de Paris 1989;65(17):1079‐82.
Colonna 1997 {published data only}
-
- Colonna L, Gonzalez‐Dunia J, Denis S. Amisulpride new data in the productive and deficit formes of schizophrenia: a review. Biological Psychiatry 1997;42(Suppl. 1):182S.
Coukell 1996 {published data only}
-
- Coukell AJ, Spencer CM, Benfield P. Amisulpride. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of schizophrenia. CNS Drugs 1996;6(3):237‐56.
Coulouvrat 1999 {published data only}
-
- Coulouvrat C, Dondey‐Nouvel L. Safety of amisulpride (Solian): a review of 11 clinical studies. International Clinical Psychopharmacology 1999;14:209‐18. - PubMed
Darondel 1983 {published data only}
-
- Darondel A, Ait‐Menguellet A, Busch M, Dupont A. Open study to determine DAN2163 postition for acute delirious psychosis [Étude ouverte du DAN2163 dans les psychoses délirantes aigües]. Rapport d'Expertise, 2163 D355, 1983.
Dollfus 1992 {published data only}
-
- Dollfus S, Petit M, Menard JF, Lesieur P. Amisulpride versus bromocriptine in infantile autism: a controlled crossover comparative study of two drugs with opposite effects on dopaminergic function. Journal of Autism and Developmental Disorders 1992;22(1):47‐60. - PubMed
-
- Dollfus S, Petit P, Menard JF. Pharmacoclinical study of an agonist and an antagonist of dopamine in early infantile autism. Neuropsychiatrie de l'enfance et de l'adolescence 1992;40(5‐6):300‐9.
Duval 1993 {published data only}
-
- Duval F, Mokrani MC, Macher JP, Crocq MA, Oliveira Castro J, Bailey P, Lataste X. Neuroendocrine profile of SDZ HDC‐912 and OPC‐4392, two new atypical antipsychotic drugs, in schizophrenic patients. Psychopharmacology 1993;110(1‐2):177‐80. - PubMed
Gayral 1981 {published data only}
-
- Gayral LF. Clinical essay of DAN2163 [Essai clinique du DAN2163]. Rapport d'Expertise, 2163 D163, 1981.
Jäger‐Becker 1996 {published data only}
-
- Jäger‐Becker D. Amisulpride improves negative symptoms in schizophrenia. TW Neurologie Psychiatrie 1996;10(10):760.
Josserand 1988 {published data only}
-
- Josserand F, Weber F. Amisulpride (Solian registered ): an energizing neuroleptic with prompt effectiveness against negative symptoms [L'Amisulpride (Solian): Un neuroleptique antidéficitaire d'action rapide]. Annales de Psychiatrie 1988;3(3 bis):306‐311.
Lecrubier 1981 {published data only}
-
- Lecrubier Y. General review. Preliminary clinical studies of DAN2163 [Revue générale. Etudes cliniques préliminaires du DAN2163]. Rapport d'expertise, 2163 D 357, 1981.
Lecrubier 1988 {published data only}
-
- Lecrubier Y, Puech A J, Aubin F, Boyer P, Deyrieux B. Comparative double‐blind study of two doses of amisulpride and placebo in the treatment of the negative syndrome of non‐psychotic subjects. In: Borenstein P editor(s). Amisulpride. Paris: Expansion Scientifique Française, 1989:167‐79.
-
- Lecrubier Y, Puech AJ, Aubin F, Boyer P, Deyrieux B. Improvement by amisulpride of the negative syndrome of non‐psychotic subjects: a preliminary study. Psychiatrie and Psychobiologie 1988;3:329‐33.
Linde 1982 {published data only}
-
- Linde OK, Mammele H, Stripp L. [Offene Studie mit DAN2163]. Psycho 1982;8:57‐8.
Mann 1984 {published data only}
-
- Mann K, Bartels M, Bauer H, Gaertner HJ. Amisulpride ‐ an open clinical study of a new benzamide in schizophrenic patients. Pharmacopsychiatry 1984;17(4):111‐5. - PubMed
Martinot 1996 {published data only}
-
- Martinot JL, Paillère‐Martinot ML, Poirier MF, Dao‐Castellana MH, Loc'h C, Mazière B. In vivo characteristics of dopamine D2 receptor occupancy by amisulpride in schizophrenia. Psychopharmacology 1996;124:154‐8. - PubMed
Maubray a 1988 {published data only}
-
- Maubray MC, Jacquot C, Ganidec J, Guez M, Idée JM, Margarit J. [Profil pharmacologique et bioclinique de l'amisulpride]. Annales de Psychiatrie 1988;3(3 bis):284‐97.
Maubray b 1988 {published data only}
-
- Maubray MC, Trouvin JH, Margarit J, Jacquot C. [Comparison de l'amisulpride (Solian) et de l'haloperidol: études comportementales et neurobiochimiques]. Réunion de l'Association des Pharmacologistes Français, Belges et Suisses, Lousanne, 23‐26 mars. 1988.
Mecheri 1993 {published data only}
-
- Mecheri G, Marie‐Cardine M, Terra JL. Open trial of amisulpride on negative symptoms of schizophrenia. Psychological Medicine 1993;25(4):347‐55.
Pichot 1986 {published data only}
-
- Pichot P, Boyer P, Dreyfus JF. Clinical effects of a new substituted benzamide (amisulpride) on the negative and positive symptoms of schizophrenia. Clinical Neuropharmacology 1986;9(Suppl. 4):115.
Rein 1995 {published data only}
-
- Rein, W. Acute and long‐term treatment of schizophrenia: different strategies for positive and negative symptoms?. 8th European College of Neuropsychopharmacology Congress; 1995; Venice, Italy. 1995.
Rein a 1996 {published data only}
-
- Rein W, Favannec‐Meidinger C, Turjanski S. Amisulpride, an "atypical" antipsychotic. Safety profile. Xth World Congress of Psychiatry; 1996; Madrid, Spain. 1996.
Rein b 1996 {published data only}
-
- Rein W, Turjanski S, Fleurot O. Amisulpride in the Treatment of Deficit Schizophrenia. Xth World Congress of Psychiatry; 1996; Madrid, Spain. 1996.
Rein c 1996 {published data only}
-
- Rein W, Turjanski S, Fleurot O. Amisulpride in the Treatment of Productive Schizophrenia. Xth World Congress of Psychiatry; 1996; Madrid, Spain. 1996.
Singer 1983 {published data only}
-
- Singer L. [Étude ouverte de l'efficacité et de la tolérance de faibles doses de DAN2163 dans le traitement des schizophrenies affectives]. Rapport d'Expertise, 2163 D354, 1983.
Singer 1990 {published data only}
-
- Singer L, Danion JM. Therapeutic value of low doses of amisulpride in the treatment of schizophrenia where negative symptoms predominate. Journal Médical de Strasbourg 1990;144(6):344‐8.
Souetre 1992 {published data only}
-
- Souêtre E, Martin P, Lecanu JP, Alexandre L, Lozet H, Gauthier JM, Camus C. Medico‐economic assessment of neuroleptics in schizophrenia. Amisulpride versus haloperidol. Encephale 1992;18(3):263‐9. - PubMed
Sutter 1983 {published data only}
-
- Sutter JM, Tatossian A. [DAN 2163: étude clinique]. Rapport d'expertise, 2163 D339, 1983.
Terra 1990 {published data only}
-
- Terra JL, Chatard JP, Dufour H, Fremont P, Gorceix A, Guedj B, Guyotat J, Leger JM, Marie‐Cardine M, Wartel R. Value of amisulpride in schizophrenia with negative symptoms. Results of an open, multicentre trial as single‐blind therapy [Intérêt de l'amisulpride dans les schizophénies à forme déficitaire. Résultats d'un essai ouvert multicentrique en monothérapie]. Semaine des Hopitaux 1990;1(66):251‐3.
Toren 1998 {published data only}
-
- Toren P, Laor N, Weizman A. Use of atypical neuroleptics in child and adolescent psychiatry. Journal of Clinical Psychiatry 1998;59(12):644‐56. - PubMed
Trichard 1998 {published data only}
-
- Trichard C, Paillère‐Martinot ML, Attar Levy D, Recassens C, Monnet F, Martinot JL. Binding of antipsychotic drugs to cortical 5‐HT(2A) receptors: A PET study of chlorpromazine, clozapine, and amisulpride in schizophrenic patients. American Journal of Psychiatry 1998;4:505‐8. - PubMed
Tridon 1989 {published data only}
-
- Tridon P. Clinical testing of amisulpride in child neuropsychiatry. Neuropsychiatrie de l'enfance et de l'adolescence 1989;37(8‐9):441‐4.
Trieloff 1996 {published data only}
-
- Trieloff I. Dose dependent mechanism of action of amisulpride in the treatment of schizophrenia. TW Neurologie Psychiatrie 1996;10(12):946.
Turjanski 1998 {published data only}
-
- Turjanski S, Rein W, Théron M. Onset of action in acute schizophrenia, amisulpride versus haloperidol. European College of Neuropsychopharmacology Congress; 1998; Paris, France. 1998.
Vaiva 1994 {published data only}
-
- Vaiva G, Thomas P, Desbonnet P, Dutoit D, Bianchi Decaix I, Goudemand M, Steinling M. Regional cerebral blood flow before and after low doses of amisulpride in negative schizophrenia. Circulation et Metabolisme du Cerveau 1994;1:21‐32.
Widlocher 1990 {published data only}
-
- Widlocher D, Allilaire JF, Guerard des Lauriers A, Lecrubier Y. [L'amisulpride, neuroleptique et antidéficitaire]. Encephale 1990;16:159‐63. - PubMed
References to studies awaiting assessment
Gray 1998 {published data only}
-
- Gray 1998. Amisulpride: a novel antipsychotic with low propensity to cause extrapyramidal side effects. Mental Health Care 1998;2(1):18‐9.
Additional references
Altman 1996
Andreasen 1983
-
- Andreasen NC. Negative symptoms in schizophrenia. Archives of General Psychiatry 1983;39:784‐8. - PubMed
Andreasen 1984
-
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS). Archives of General Psychiatry. Iowa City: The University of Iowa, 1984.
Barnes 1989
-
- Barnes TR. A rating scale for drug‐induced akathisia. British Journal of Psychiatry 1989;154:672‐6. - PubMed
Begg 1996
-
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of randomized controlled trials. The CONSORT statement. JAMA 1996;276:637‐9. - PubMed
Boyer 1995
-
- Boyer P, Lecrubier Y, Puech AJ, Dewailly J, Aubin F. Treatment of negative symtoms in schizophrenia with amisulpride. British Journal of Psychiatry 1995;166(1):68‐72. - PubMed
Endicott 1976
-
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry 1976;33(6):766‐71. - PubMed
Freeman 1997
-
- Freeman HL. Amisulpride compared with standad neuroleptics in acute exacerbations of schizophrenia: three efficacy studies. International Clinical Psychopharmacology 1997;12(2):S11‐S17. - PubMed
Guy 1976
-
- Guy W. Early clinical drug evaluation (ECDEU) assessment manual for psychopharmacology. Psychopharmacology Research Branch. Washington, DC: National Institute of Mental Health, 1976:217‐222 217‐222 217‐222.
Honigfeld 1965
-
- Honigfeld G, Klett CJ. The nurses' observation scale for inpatient evaluation. A new scale for measuring improvement in chronic schizophrenia. Journal of Clinical Psychology 1965;21:65‐71. - PubMed
Jadad 1996
-
- Jadad A, Moore A, Carroll D, Jenkinson C, Reynolds DJM, Gavanagh DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. - PubMed
Kay 1987
-
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophrenia Bulletin 1987;13:261‐7. - PubMed
Krawiecka 1977
-
- Krawiecka M, Goldberg D, Vaughan M. A standardised psychiatric assessment scale for rating chronic psychotic patients. Acta Psychiatrica Scandinavica 1977;35:299‐308. - PubMed
Montgomery 1979
-
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. - PubMed
Mulrow 1997
-
- Mulrow CD, Oxman AD (eds). Cochrane Collaboration Handbook [updated 1 March 1997]. The Cochrane Library [database on disk and CDROM]. The Cochrane Collaboration 1997, Issue 1.
NIMH 1975
-
- National Institute of Mental Health. Psychopharmacology Research Branch. Development of a dyskinetic movement scale. Early Clinical Drug Evaluation Unit. Interco 1975;4:3‐6.
Overall 1962
-
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports 1962;10:799‐812.
Rein 1997
-
- Rein W, Turjanski S. Clinical update on amisulpride in deficit schizophrenia. International Clinical Psychopharmacology 1997;12(2):S19‐27. - PubMed
Simpson 1970
-
- Simpson EN, Angus JWF. A rating scale for extrapyramidal side‐effects. Acta Psychiatrica Scandinavica Supplementum 1970;212:11‐9. - PubMed
Souetre 1992
-
- Souetre E, Martin P, Lecanu JP, et al. Medico‐economic assessment of neuroleptics in schizophrenia. Amisulpride versus haloperidol. Encephale 1992;18(3):263‐9. - PubMed
Thornley 1998
-
- Thornley B, Adams CE, Awad G. Chlorpromazine versus placebo for those with schizophrenia (Cochrane Review). The Cochrane Library 1998, Issue 4.
Wahlbeck 1998
-
- Wahlbeck K, Cheine M, Essali MA. Clozapine versus 'typical' neuroleptic medication for schizophrenia (Cochrane Review). The Cochrane Library 1998, Issue 4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

