Cidofovir in the treatment of poxvirus infections
- PMID: 12076747
- PMCID: PMC9533828
- DOI: 10.1016/s0166-3542(02)00008-6
Cidofovir in the treatment of poxvirus infections
Abstract
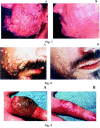
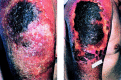
Cidofovir [(S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine, HPMPC] has since 1996 been licensed for clinical use in the treatment of cytomegalovirus (CMV) retinitis in AIDS patients. Cidofovir has broad-spectrum activity against virtually all DNA viruses, including herpes-, adeno-, polyoma-, papilloma- and poxviruses. Among the poxviruses, vaccinia, variola (smallpox), cowpox, monkeypox, camelpox, molluscum contagiosum and orf have proven sensitive to the inhibitory effects of cidofovir. In vivo, cidofovir has shown high efficacy, even after administration of a single systemic (intraperitoneal) or intranasal (aerosolized) dose, in protecting mice from a lethal respiratory infection with either vaccinia or cowpox. Cidofovir has also demonstrated high effectiveness in the treatment of vaccinia virus infection in severe combined immune deficiency mice. In humans, cidofovir has been used successfully in the treatment, by both the topical and intravenous route, of recalcitrant molluscum contagiosum and orf in immunocompromised patients. Taken together, these data indicate that cidofovir should be effective in the therapy and short-term prophylaxis of smallpox and related poxvirus infections in humans, as well as the treatment of the complications of vaccinia that may arise in immunocompromised patients inadvertently inoculated with the smallpox vaccine (vaccinia).
Figures
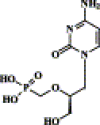
 ) represents a phosphonate group, whereas (
) represents a phosphonate group, whereas ( ) corresponds to a phosphate group.
) corresponds to a phosphate group.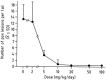
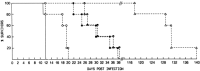
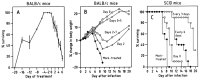
 ) (n=5); or at 20 mg/kg/twice a week for up to 20 weeks (▵) (n=5) (Neyts and De Clercq, 1993).
) (n=5); or at 20 mg/kg/twice a week for up to 20 weeks (▵) (n=5) (Neyts and De Clercq, 1993).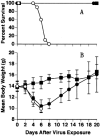



References
-
- Andrei G., Snoeck R., Schols D., De Clercq E. Induction of apoptosis by cidofovir in human papillomavirus (HPV)-positive cells. Oncol. Res. 2001;12:397–408. - PubMed
-
- Bray M., Martinez M., Smee D.F., Kefauver D., Thompson E., Huggins J.W. Cidofovir protects mice against lethal aerosol or intranasal cowpox virus challenge. J. Infect. Dis. 2000;181:10–19. - PubMed
-
- Cihlar T., Chen M.S. Identification of enzymes catalyzing two-step phosphorylation of cidofovir and the effect of cytomegalovirus infection on their activities in host cells. Mol. Pharmacol. 1996;50:1502–1510. - PubMed
-
- Cihlar T., Votruba I., Horská K., Liboska R., Rosenberg I., Holý A. Metabolism of 1-(S)-3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) in human embryonic lung cells. Collect. Czech. Chem. Commun. 1992;57:661–672.
-
- Cochereau I., Doan S., Diraison M.-C., Mousalatti H., Guvenisik N., Ren L., Hoang-Xuan T. Uveitis in patients treated with intravenous cidofovir. Ocul. Immunol. Inflamm. 1999;7:223–229. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

