The association between neuronal nitric oxide synthase and neuronal sensitivity in the brain after brain injury
- PMID: 12076978
- PMCID: PMC2751793
- DOI: 10.1111/j.1749-6632.2002.tb04071.x
The association between neuronal nitric oxide synthase and neuronal sensitivity in the brain after brain injury
Abstract
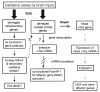
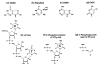
Injury to the central nervous system is the leading cause of disability in the United States. Neuronal death is one of the causes of disability. Among patients who survive this type of injury, various degrees of recovery in brain function are observed. The molecular basis of functional recovery is poorly understood. Clinical observations and research using experimental injury models have implicated several metabolites in the cascade of events that lead to neuronal degeneration. The levels of intracellular ATP (energy source) and pH are decreased, whereas levels of extracellular glutamate, intracellular calcium ions, and oxidative damage to RNA/DNA, protein, and lipid are increased. These initiating events can be associated with energy failure and mitochondrial dysfunction, resulting in functional or structural brain damage. The injured brain is known to express immediate early genes. Recent studies show that reactive oxygen species (ROS) cause lesions in genes from which mRNA is transcribed as part of the endogenous neuroprotective response. Although degenerating proteins and lipids may contribute to necrosis significantly after severe injury, abnormalities in genetic material, if not repaired, disturb cellular function at every level by affecting replication, transcription, and translation. These lesions include abnormal nucleic acids, known as oxidative lesions of DNA (ODLs) or of RNA (ORLs). In this review, we focus on our current understanding of the various effects of neuronal nitric oxide synthase on the formation of modified bases in DNA and RNA that are induced in the brain after injury, and how ODLs and ORLs affect cell function.
Figures
References
-
- Lipton P. Ischemic cell death in brain neurons. Physiol. Rev. 1999;79:1431–1568. - PubMed
-
- Lewen A, et al. Free radical pathways in CNS injury. J. Neurotrauma. 2000;17:871–890. - PubMed
-
- Eliasson MJ, et al. Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat. Med. 1997;3:1089–1095. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

