Increase in the pool size of releasable synaptic vesicles by the activation of protein kinase C in goldfish retinal bipolar cells
- PMID: 12077174
- PMCID: PMC6757743
- DOI: 10.1523/JNEUROSCI.22-12-04776.2002
Increase in the pool size of releasable synaptic vesicles by the activation of protein kinase C in goldfish retinal bipolar cells
Abstract
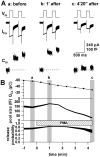
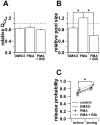
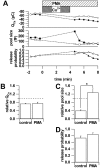
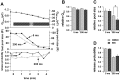
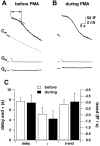
Secretion from neurons and neuroendocrine cells is enhanced by the activation of protein kinase C (PKC) in various preparations. We have already reported that transmitter (glutamate) release from Mb1 bipolar cells in the goldfish retina is potentiated by the activation of PKC. However, it is not yet settled whether the potentiation is ascribed to the increase in the pool size of releasable synaptic vesicles or in release probability. In the present study, Ca2+ influx and exocytosis were simultaneously monitored by measuring the presynaptic Ca2+ current and membrane capacitance changes, respectively, in a terminal detached from the bipolar cell. The double pulse protocol was used to estimate separately the changes in the pool size and release probability. The activation of PKC by phorbol 12-myristate 13-acetate (PMA) specifically increased the pool size but not the release probability. PKC was activated by PMA even after the Ca2+ influx was blocked by Co2+. In bipolar cells the releasable pool can be divided into two components: one is small and rapidly exhausted, and the other is large and slowly exocytosed. To identify which component is responsible for the increase in the pool size, the effects of PMA and a PKC-specific inhibitor, bisindolylmaleimide I (BIS), on each component were examined. The slow component was selectively increased by PMA and reduced by BIS. Thus, we conclude that the activation of PKC in Mb1 bipolar cells potentiates glutamate release by increasing the pool size of the slow component.
Figures


Similar articles
-
Potentiation of transmitter release by protein kinase C in goldfish retinal bipolar cells.J Physiol. 1998 Oct 1;512 ( Pt 1)(Pt 1):219-25. doi: 10.1111/j.1469-7793.1998.219bf.x. J Physiol. 1998. PMID: 9729631 Free PMC article.
-
Regulation of transmitter release from retinal bipolar cells.Prog Biophys Mol Biol. 1999;72(2):109-33. doi: 10.1016/s0079-6107(99)00003-6. Prog Biophys Mol Biol. 1999. PMID: 10511797
-
Two components of transmitter release in retinal bipolar cells: exocytosis and mobilization of synaptic vesicles.Neurosci Res. 1997 Apr;27(4):357-70. doi: 10.1016/s0168-0102(97)01168-1. Neurosci Res. 1997. PMID: 9152048
-
Electrophysiology of synaptic vesicle cycling.Annu Rev Physiol. 1999;61:725-52. doi: 10.1146/annurev.physiol.61.1.725. Annu Rev Physiol. 1999. PMID: 10099708 Review.
-
Spatio-temporal regulation of neurotransmitter release by PKC; studies in adrenal chromaffin cells.Crit Rev Neurobiol. 2004;16(1-2):173-9. doi: 10.1615/critrevneurobiol.v16.i12.180. Crit Rev Neurobiol. 2004. PMID: 15581412 Review.
Cited by
-
Synaptic mechanisms of adaptation and sensitization in the retina.Nat Neurosci. 2013 Jul;16(7):934-41. doi: 10.1038/nn.3408. Epub 2013 May 19. Nat Neurosci. 2013. PMID: 23685718 Free PMC article.
-
Phorbol esters target the activity-dependent recycling pool and spare spontaneous vesicle recycling.J Neurosci. 2005 Nov 23;25(47):10922-9. doi: 10.1523/JNEUROSCI.3766-05.2005. J Neurosci. 2005. PMID: 16306405 Free PMC article.
-
The Effect of PKCα on the Light Response of Rod Bipolar Cells in the Mouse Retina.Invest Ophthalmol Vis Sci. 2015 Jul;56(8):4961-74. doi: 10.1167/iovs.15-16622. Invest Ophthalmol Vis Sci. 2015. PMID: 26230760 Free PMC article.
-
Specificity emerges in the dissection of diacylglycerol- and protein kinase C-mediated signalling pathways.Proc Natl Acad Sci U S A. 2002 Dec 24;99(26):16522-3. doi: 10.1073/pnas.022708199. Epub 2002 Dec 16. Proc Natl Acad Sci U S A. 2002. PMID: 12486246 Free PMC article. No abstract available.
-
Distinct roles of different neural cell adhesion molecule (NCAM) isoforms in synaptic maturation revealed by analysis of NCAM 180 kDa isoform-deficient mice.J Neurosci. 2004 Feb 25;24(8):1852-64. doi: 10.1523/JNEUROSCI.4406-03.2004. J Neurosci. 2004. PMID: 14985425 Free PMC article.
References
-
- Asaoka Y, Nakamura S, Yoshida K, Nishizuka Y. Protein kinase C, calcium and phospholipid degradation. Trends Biochem Sci. 1992;17:414–417. - PubMed
-
- Betz A, Ashery U, Rickmann M, Augustin I, Neher E, Südhof TC, Rettig J, Brose N. Munc13–1 is a presynaptic phorbol ester receptor that enhances neurotransmitter release. Neuron. 1998;21:123–136. - PubMed
-
- Bowlby MR, Levitan IB. Block of cloned voltage-gated potassium channels by the second messenger diacylglycerol independent of protein kinase C. J Neurophysiol. 1995;73:2221–2229. - PubMed
-
- Brose N, Rosenmund C, Rettig J. Regulation of transmitter release by Unc-13 and its homologues. Curr Opin Neurobiol. 2000;10:303–311. - PubMed
-
- Castagna M, Takai Y, Kaibuchi K, Sano K, Kikkawa U, Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982;257:7847–7851. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
