P2X receptor trafficking in neurons is subunit specific
- PMID: 12077178
- PMCID: PMC6757758
- DOI: 10.1523/JNEUROSCI.22-12-04814.2002
P2X receptor trafficking in neurons is subunit specific
Abstract
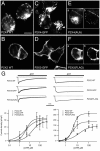
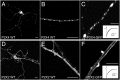
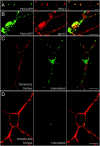
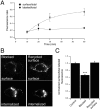
P2X receptors within the CNS mediate excitatory synaptic transmission and also act presynaptically to modulate neurotransmitter release. We have studied the targeting and trafficking of P2X4 and P2X2 receptors heterologously expressed in cultured olfactory bulb neurons. Homomeric P2X4 receptors had a punctate distribution, and many of the puncta colocalized with early endosomes. In contrast, P2X2 receptors were primarily localized at the plasma membrane. By antibody-labeling of surface receptors in living neurons, we showed that P2X4 receptors undergo rapid constitutive internalization and subsequent reinsertion into the plasma membrane, whereas P2X2 receptors were not regulated in such a way. The internalization of P2X4 receptors was dynamin-dependent, and the binding of ATP enhanced the basal rate of retrieval in a Ca2+-independent manner. The presence of the P2X4 subunit in a P2X4/6 heteromer governed the trafficking properties of the receptor. P2X receptors acted presynaptically to enhance the release of glutamate, suggesting that the regulated cycling of P2X4-containing receptors might provide a mechanism for modulation of synaptic transmission.
Figures

Similar articles
-
Selective potentiation of homomeric P2X2 ionotropic ATP receptors by a fast non-genomic action of progesterone.Neuropharmacology. 2010 Mar;58(3):569-77. doi: 10.1016/j.neuropharm.2009.12.002. Epub 2009 Dec 16. Neuropharmacology. 2010. PMID: 20004677
-
Cross-inhibition between nicotinic acetylcholine receptors and P2X receptors in myenteric neurons and HEK-293 cells.Am J Physiol Gastrointest Liver Physiol. 2009 Jun;296(6):G1267-76. doi: 10.1152/ajpgi.00048.2009. Epub 2009 Apr 2. Am J Physiol Gastrointest Liver Physiol. 2009. PMID: 19342512 Free PMC article.
-
Characterization of cultured dorsal root ganglion neuron P2X receptors.Eur J Neurosci. 1999 Jan;11(1):149-54. doi: 10.1046/j.1460-9568.1999.00426.x. Eur J Neurosci. 1999. PMID: 9987019
-
Pharmacology of cloned P2X receptors.Annu Rev Pharmacol Toxicol. 2000;40:563-80. doi: 10.1146/annurev.pharmtox.40.1.563. Annu Rev Pharmacol Toxicol. 2000. PMID: 10836147 Review.
-
Molecular physiology of P2X receptors.Physiol Rev. 2002 Oct;82(4):1013-67. doi: 10.1152/physrev.00015.2002. Physiol Rev. 2002. PMID: 12270951 Review.
Cited by
-
P2X4 purinoceptor signaling in chronic pain.Purinergic Signal. 2012 Sep;8(3):621-8. doi: 10.1007/s11302-012-9306-7. Epub 2012 Apr 15. Purinergic Signal. 2012. PMID: 22528681 Free PMC article. Review.
-
Implication of Neuronal Versus Microglial P2X4 Receptors in Central Nervous System Disorders.Neurosci Bull. 2020 Nov;36(11):1327-1343. doi: 10.1007/s12264-020-00570-y. Epub 2020 Sep 5. Neurosci Bull. 2020. PMID: 32889635 Free PMC article. Review.
-
P2X4 receptor re-sensitization depends on a protonation/deprotonation cycle mediated by receptor internalization and recycling.J Physiol. 2018 Oct;596(20):4893-4907. doi: 10.1113/JP275448. Epub 2018 Sep 17. J Physiol. 2018. PMID: 30144063 Free PMC article.
-
P2X-GCaMPs as Versatile Tools for Imaging Extracellular ATP Signaling.eNeuro. 2021 Jan 28;8(1):ENEURO.0185-20.2020. doi: 10.1523/ENEURO.0185-20.2020. Print 2021 Jan-Feb. eNeuro. 2021. PMID: 33380526 Free PMC article.
-
Trimerisation is important for the function of clathrin at the mitotic spindle.J Cell Sci. 2006 Oct 1;119(Pt 19):4071-8. doi: 10.1242/jcs.03192. Epub 2006 Sep 12. J Cell Sci. 2006. PMID: 16968737 Free PMC article.
References
-
- Beattie EC, Carroll RC, Yu X, Morishita W, Yasuda H, von Zastrow M, Malenka RC. Regulation of AMPA receptor endocytosis by a signaling mechanism shared with LTD. Nat Neurosci. 2000;3:1291–1300. - PubMed
-
- Burrone J, Murthy VN. Synaptic plasticity: rush hour traffic in the AMPA lanes. Curr Biol. 2001;11:R274–R277. - PubMed
-
- Carroll RC, Beattie EC, von Zastrow M, Malenka RC. Role of AMPA receptor endocytosis in synaptic plasticity. Nat Rev Neurosci. 2001;2:315–324. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
