A novel function of monomeric amyloid beta-protein serving as an antioxidant molecule against metal-induced oxidative damage
- PMID: 12077180
- PMCID: PMC6757724
- DOI: 10.1523/JNEUROSCI.22-12-04833.2002
A novel function of monomeric amyloid beta-protein serving as an antioxidant molecule against metal-induced oxidative damage
Abstract
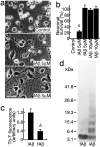
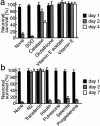
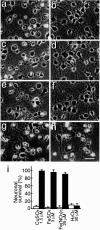
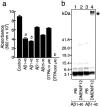
Aggregated and oligomeric amyloid beta-protein (Abeta) is known to exhibit neurotoxicity. However, the action of Abeta monomers on neurons is not fully understood. We have studied aggregation state-dependent actions of Abeta and found an oligomer-specific effect of Abeta on lipid metabolism in neurons (Michikawa et al., 2001). Here, we show a novel function of monomeric Abeta1-40, which is the major species found in physiological fluid, as a natural antioxidant molecule that prevents neuronal death caused by transition metal-induced oxidative damage. Monomeric Abeta1-40, which is demonstrated by SDS-PAGE after treatment with glutaraldehyde, protects neurons cultured in a medium containing 1.5 microm Fe(II) without antioxidant molecules. Metal ion chelators such as EDTA, CDTA (trans-1,2-diaminocyclohexane-N,N,N',N'-tetraacetic acid), and DTPA (diethylenetriamine-N,N,N',N",N"-penta-acetic acid, an iron-binding protein, transferrin, and antioxidant scavengers such as catalase, glutathione, and vitamin E also inhibit neuronal death under the same conditions. Monomeric Abeta1-40 inhibits neuronal death caused by Cu(II), Fe(II), and Fe(III) but does not protect neurons against H2O2-induced damage. Monomeric Abeta1-40 inhibits the reduction of Fe(III) induced by vitamin C and the generation of superoxides and prevents lipid peroxidation induced by Fe(II). Abeta1-42 remaining as a monomer also exhibits antioxidant and neuroprotective effects. In contrast, oligomeric and aggregated Abeta1-40 and Abeta1-42 lose their neuroprotective activity. These results indicate that monomeric Abeta protects neurons by quenching metal-inducible oxygen radical generation and thereby inhibiting neurotoxicity. Because aggregated Abeta is known to be an oxygen radical generator, our results provide a novel concept that the aggregation-dependent biological effects of Abeta are dualistic, being either an oxygen radical generator or its inhibitor.
Figures

Similar articles
-
Amyloid beta-protein (Abeta)1-40 protects neurons from damage induced by Abeta1-42 in culture and in rat brain.J Neurochem. 2003 Nov;87(3):609-19. doi: 10.1046/j.1471-4159.2003.02018.x. J Neurochem. 2003. PMID: 14535944
-
Ferulic acid ethyl ester protects neurons against amyloid beta- peptide(1-42)-induced oxidative stress and neurotoxicity: relationship to antioxidant activity.J Neurochem. 2005 Feb;92(4):749-58. doi: 10.1111/j.1471-4159.2004.02899.x. J Neurochem. 2005. PMID: 15686476
-
Protective effects of vitamin E against oxidative damage induced by Abeta1-40Cu(II) complexes.Acta Biochim Biophys Sin (Shanghai). 2007 Feb;39(2):123-30. doi: 10.1111/j.1745-7270.2007.00261.x. Acta Biochim Biophys Sin (Shanghai). 2007. PMID: 17277887
-
Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer's disease brain. A review.Free Radic Res. 2002 Dec;36(12):1307-13. doi: 10.1080/1071576021000049890. Free Radic Res. 2002. PMID: 12607822 Review.
-
The redox chemistry of the Alzheimer's disease amyloid beta peptide.Biochim Biophys Acta. 2007 Aug;1768(8):1976-90. doi: 10.1016/j.bbamem.2007.02.002. Epub 2007 Feb 9. Biochim Biophys Acta. 2007. PMID: 17433250 Review.
Cited by
-
Decreased plasma C-reactive protein levels in APOE ε4 allele carriers.Ann Clin Transl Neurol. 2018 Sep 17;5(10):1229-1240. doi: 10.1002/acn3.639. eCollection 2018 Oct. Ann Clin Transl Neurol. 2018. PMID: 30349858 Free PMC article.
-
Staying connected: synapses in Alzheimer disease.Am J Pathol. 2004 Nov;165(5):1461-4. doi: 10.1016/S0002-9440(10)63404-9. Am J Pathol. 2004. PMID: 15509517 Free PMC article. Review. No abstract available.
-
Effect of C-terminal residues of Aβ on copper binding affinity, structural conversion and aggregation.PLoS One. 2014 Mar 3;9(3):e90385. doi: 10.1371/journal.pone.0090385. eCollection 2014. PLoS One. 2014. PMID: 24594588 Free PMC article.
-
The Alzheimer's amyloid β-peptide (Aβ) binds a specific DNA Aβ-interacting domain (AβID) in the APP, BACE1, and APOE promoters in a sequence-specific manner: characterizing a new regulatory motif.Gene. 2011 Nov 15;488(1-2):1-12. doi: 10.1016/j.gene.2011.06.004. Epub 2011 Jun 15. Gene. 2011. PMID: 21699964 Free PMC article.
-
Review: cell cycle aberrations and neurodegeneration.Neuropathol Appl Neurobiol. 2010 Apr;36(2):157-63. doi: 10.1111/j.1365-2990.2010.01064.x. Epub 2010 Jan 6. Neuropathol Appl Neurobiol. 2010. PMID: 20059701 Free PMC article. Review.
References
-
- Atwood CS, Moir RD, Huang X, Scarpa RC, Bacarra NM, Romano DM, Hartshorn MA, Tanzi RE, Bush AI. Dramatic aggregation of Alzheimer Aβ by Cu(II) is induced by conditions representing physiological acidosis. J Biol Chem. 1998;273:12817–12826. - PubMed
-
- Atwood CS, Scarpa RC, Huang X, Moir RD, Jones WD, Fairlie DP, Tanzi RE, Bush AI. Characterization of copper interactions with alzheimer amyloid β peptides: identification of an attomolar-affinity copper binding site on amyloid β1–42. J Neurochem. 2000;75:1219–1233. - PubMed
-
- Behl C, Davis JB, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid β-protein toxicity. Cell. 1994;77:817–827. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
