Control and plasticity of intercellular calcium waves in astrocytes: a modeling approach
- PMID: 12077182
- PMCID: PMC6757753
- DOI: 10.1523/JNEUROSCI.22-12-04850.2002
Control and plasticity of intercellular calcium waves in astrocytes: a modeling approach
Abstract
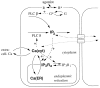
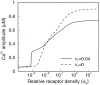
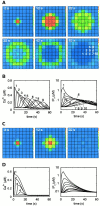
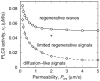
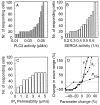
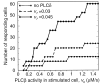
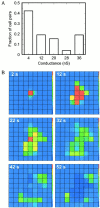
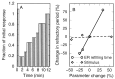
Intercellular Ca2+ waves in astrocytes are thought to serve as a pathway of long-range signaling. The waves can propagate by the diffusion of molecules through gap junctions and across the extracellular space. In rat striatal astrocytes, the gap-junctional route was shown to be dominant. To analyze the interplay of the processes involved in wave propagation, a mathematical model of this system has been developed. The kinetic description of Ca2+ signaling within a single cell accounts for inositol 1,4,5-trisphosphate (IP3) generation, including its activation by cytoplasmic Ca2+, IP3-induced Ca2+ liberation from intracellular stores and various other Ca2+ transports, and cytoplasmic diffusion of IP3 and Ca2+. When cells are coupled by gap junction channels in a two-dimensional array, IP3 generation in one cell triggers Ca2+ waves propagating across some tens of cells. The spatial range of wave propagation is limited, yet depends sensitively on the Ca2+-mediated regeneration of the IP3 signal. Accordingly, the term "limited regenerative signaling" is proposed. The gap-junctional permeability for IP3 is the crucial permissive factor for wave propagation, and heterogeneity of gap-junctional coupling yields preferential pathways of wave propagation. Processes involved in both signal initiation (activation of IP3 production caused by receptor agonist) and regeneration (activation of IP3 production by Ca2+, loading of the Ca2+ stores) are found to exert the main control on the wave range. The refractory period of signaling strongly depends on the refilling kinetics of the Ca2+ stores. Thus the model identifies multiple steps that may be involved in the regulation of this intercellular signaling pathway.
Figures
References
-
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. - PubMed
-
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium response curves of Ins(1, 4, 5)P3 and calcium gated channels from endoplasmic reticulum of the cerebellum. Nature. 1991;351:751–754. - PubMed
-
- Blomstrand F, Giaume C, Hansson E, Ronnback L. Distinct pharmacological properties of ET-1 and ET-3 on astroglial gap junctions and Ca2+ signaling. Am J Physiol. 1999;277:C616–627. - PubMed
-
- Braet K, Paemeleire K, D'Herde K, Sanderson MJ, Leybaert L. Astrocyte-endothelial cell calcium signals conveyed by two signalling pathways. Eur J Neurosci. 2001;13:79–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
