Cyclin-dependent kinase 5 phosphorylates disabled 1 independently of Reelin signaling
- PMID: 12077184
- PMCID: PMC6757745
- DOI: 10.1523/JNEUROSCI.22-12-04869.2002
Cyclin-dependent kinase 5 phosphorylates disabled 1 independently of Reelin signaling
Abstract
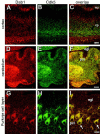
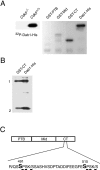
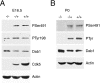
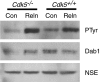
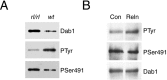
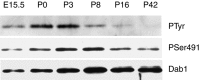
Two major signaling pathways that control neuronal positioning during brain development have been uncovered as a result of genetic and biochemical studies on neurological mouse mutants. Mice deficient in Reelin, Disabled 1 (Dab1), or both the very low-density lipoprotein receptor (VLDLR) and the apolipoprotein E receptor 2 (ApoER2) exhibit identical neuroanatomic defects in laminar structures throughout the brain. These proteins function as components of the Reelin signaling pathway. Reelin is a secreted glycoprotein that binds to VLDLR and ApoER2, inducing tyrosine phosphorylation of Dab1, an intracellular adapter protein. Neuronal migration is also regulated by cyclin-dependent kinase 5 (Cdk5) and its activating subunits p35 and p39. Mice deficient in Cdk5, p35, or both p35 and p39 exhibit lamination defects that are similar but not identical to those observed in mice with a defect in the Reelin signaling pathway. Cdk5 phosphorylates proteins that maintain cytoskeletal structures and promote cell motility. To explore the possibility that Cdk5 influences the Reelin pathway, we sought to determine whether Dab1 is a substrate for Cdk5. Here we show that Cdk5 phosphorylates Dab1 on serine 491 in vitro and in vivo, independently of Reelin signaling. We also show that ectopic neurons in Cdk5-deficient mice exhibit reduced levels of Reelin signaling during later stages of cortical development, although Cdk5 is not required for Reelin-induced tyrosine phosphorylation of Dab1. Although the functional significance of Dab1 serine phosphorylation is unclear, our results suggest that there is biochemical cross-talk between two signaling pathways that control cell positioning.
Figures


References
-
- Chae T, Kwon YT, Bronson R, Dikkes P LiE, Tsai LH. Mice lacking p35, a neuronal specific activator of Cdk5, display cortical lamination defects, seizures, and adult lethality. Neuron. 1997;18:29–42. - PubMed
-
- D'Arcangelo G, Curran T. Reeler: new tales on an old mutant mouse. BioEssays. 1998;20:235–244. - PubMed
-
- D'Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. - PubMed
-
- D'Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
