Light response of retinal ON bipolar cells requires a specific splice variant of Galpha(o)
- PMID: 12077185
- PMCID: PMC6757744
- DOI: 10.1523/JNEUROSCI.22-12-04878.2002
Light response of retinal ON bipolar cells requires a specific splice variant of Galpha(o)
Abstract
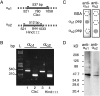
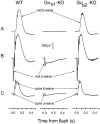
Glutamate released onto retinal ON bipolar neurons binds to a metabotropic receptor to activate a heterotrimeric G-protein (G(o)) that ultimately closes a nonspecific cation channel. Signaling requires the alpha subunit (Galpha(o)), but its effector is unknown. Because Galpha(o) is transcribed into two splice variants (alpha(o1) and alpha(o2)) that differ in the key GTPase domain, the next step in elucidating this pathway was to determine which splice variant carries the signal. Here we show by reverse transcription-PCR and Western blots that retina expresses both splice variants. Furthermore, in situ hybridization and immunostaining on mouse retina deficient in one splice variant or the other show that both alpha(o1) and alpha(o2) are expressed by ON bipolar cells but that alpha(o1) is much more abundant. Finally, electroretinography performed on mice deficient for one splice variant or the other shows that the positive b-wave (response of ON bipolar cells to rod and cone input) requires alpha(o1) but not alpha(o2). Thus, the light response of the ON bipolar cell is probably carried by its strongly expressed splice variant, Galpha(o1).
Figures




Similar articles
-
The light response of ON bipolar neurons requires G[alpha]o.J Neurosci. 2000 Dec 15;20(24):9053-8. doi: 10.1523/JNEUROSCI.20-24-09053.2000. J Neurosci. 2000. PMID: 11124982 Free PMC article.
-
Coordinated control of sensitivity by two splice variants of Gα(o) in retinal ON bipolar cells.J Gen Physiol. 2010 Oct;136(4):443-54. doi: 10.1085/jgp.201010477. Epub 2010 Sep 13. J Gen Physiol. 2010. PMID: 20837674 Free PMC article.
-
G protein subunit G gamma 13 is coexpressed with G alpha o, G beta 3, and G beta 4 in retinal ON bipolar cells.J Comp Neurol. 2003 Jan 1;455(1):1-10. doi: 10.1002/cne.10396. J Comp Neurol. 2003. PMID: 12454992
-
Expression of genes encoding glutamate receptors and transporters in rod and cone bipolar cells of the primate retina determined by single-cell polymerase chain reaction.Mol Vis. 2007 Nov 28;13:2194-208. Mol Vis. 2007. PMID: 18087239
-
Gβ3 is required for normal light ON responses and synaptic maintenance.J Neurosci. 2012 Aug 15;32(33):11343-55. doi: 10.1523/JNEUROSCI.1436-12.2012. J Neurosci. 2012. PMID: 22895717 Free PMC article.
Cited by
-
mGluR6 deletion renders the TRPM1 channel in retina inactive.J Neurophysiol. 2012 Feb;107(3):948-57. doi: 10.1152/jn.00933.2011. Epub 2011 Nov 30. J Neurophysiol. 2012. PMID: 22131384 Free PMC article.
-
Arginyltransferase (Ate1) regulates the RGS7 protein level and the sensitivity of light-evoked ON-bipolar responses.Sci Rep. 2021 Apr 30;11(1):9376. doi: 10.1038/s41598-021-88628-3. Sci Rep. 2021. PMID: 33931669 Free PMC article.
-
Molecular mechanisms underlying selective synapse formation of vertebrate retinal photoreceptor cells.Cell Mol Life Sci. 2020 Apr;77(7):1251-1266. doi: 10.1007/s00018-019-03324-w. Epub 2019 Oct 4. Cell Mol Life Sci. 2020. PMID: 31586239 Free PMC article. Review.
-
Deletion of Go2alpha abolishes cocaine-induced behavioral sensitization by disturbing the striatal dopamine system.FASEB J. 2008 Oct;22(10):3736-46. doi: 10.1096/fj.08-111245. Epub 2008 Jul 7. FASEB J. 2008. PMID: 18606864 Free PMC article.
-
G-protein-mediated inhibition of the Trp channel TRPM1 requires the Gβγ dimer.Proc Natl Acad Sci U S A. 2012 May 29;109(22):8752-7. doi: 10.1073/pnas.1117433109. Epub 2012 May 14. Proc Natl Acad Sci U S A. 2012. PMID: 22586107 Free PMC article.
References
-
- Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem. 1992;267:13361–13668. - PubMed
-
- Boycott BB, Wässle H. Morphological classification of bipolar cells of the primate retina. Eur J Neurosci. 1991;3:1069–1088. - PubMed
-
- Breton ME, Schueller AW, Lamb TD, Pugh EN., Jr Analysis of ERG a-wave amplification and kinetics in terms of the G-protein cascade of phototransduction. Invest Ophthalmol Vis Sci. 1994;35:295–309. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
