Postural modifications and neuronal excitability changes induced by a short-term serotonin depletion during neonatal development in the rat
- PMID: 12077206
- PMCID: PMC6757731
- DOI: 10.1523/JNEUROSCI.22-12-05108.2002
Postural modifications and neuronal excitability changes induced by a short-term serotonin depletion during neonatal development in the rat
Abstract
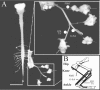
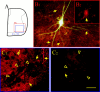
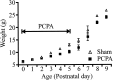
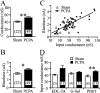
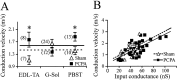
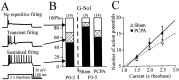
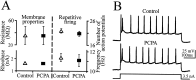
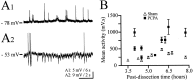
Serotonin (5-HT) plays an important role both in the development and in the recovery of locomotion after spinalization in vertebrates. We investigated the contribution of the serotonergic system to the maturation of the lumbar motoneurons and networks in the neonatal rat. A 5-HT synthesis inhibitor, p-chlorophenylalanine (PCPA), was administered daily from the first postnatal day (P0) onward. This protocol depleted serotonin in the spinal cord within 3-4 d, as demonstrated by immunohistochemistry. PCPA-treated rats exhibited postural changes characterized by lesser flexion at the knee and ankle levels and lesser extension of the hip. Posture was asymmetric, suggesting possible deficits in the interlimb coordination. Intracellular recordings were made at P3-5 from motoneurons innervating different hindlimb muscles, using the in vitro brainstem-spinal cord-nerve-attached preparation. In PCPA-treated rats, the conduction velocity of motoneurons was increased, and their excitability was decreased (because of higher rehobase and input conductance) compared with sham animals. In accordance with postural observations, changes were more pronounced in hip extensor/knee flexor than in ankle extensor motoneurons. The maturation of repetitive firing properties was stopped by PCPA treatment, although PCPA, applied in vitro, had no effect on membrane properties. The spontaneous endogenously generated activity, which is a characteristic of immature networks, was increased in PCPA-treated rats, suggesting that developing lumbar networks are sensitive to 5-HT levels. Serotonin may play a critical role during development in regulating the balance between the excitability of motoneurons and that of interneurons. Interneuronal excitability is crucial for the activity-dependent development of spinal cord networks.
Figures

Similar articles
-
Differential maturation of motoneurons innervating ankle flexor and extensor muscles in the neonatal rat.Eur J Neurosci. 2000 Dec;12(12):4562-6. doi: 10.1046/j.0953-816x.2000.01321.x. Eur J Neurosci. 2000. PMID: 11122369
-
Serotonin refines the locomotor-related alternations in the in vitro neonatal rat spinal cord.Eur J Neurosci. 2005 Mar;21(5):1338-46. doi: 10.1111/j.1460-9568.2005.03971.x. Eur J Neurosci. 2005. PMID: 15813943
-
Prenatal administration of para-chlorophenylalanine results in suppression of serotonergic system and disturbance of swimming movements in newborn rats.Neurosci Res. 1998 Jun;31(2):155-69. doi: 10.1016/s0168-0102(98)00034-0. Neurosci Res. 1998. PMID: 9700721
-
Perinatal development of the motor systems involved in postural control.Neural Plast. 2005;12(2-3):131-9; discussion 263-72. doi: 10.1155/np.2005.131. Neural Plast. 2005. PMID: 16097481 Free PMC article. Review.
-
Contribution of postural muscle tone to full expression of posture and locomotor movements: multi-faceted analyses of its setting brainstem-spinal cord mechanisms in the cat.Jpn J Physiol. 1989;39(6):785-809. Jpn J Physiol. 1989. PMID: 2698966 Review.
Cited by
-
A dynamic role for dopamine receptors in the control of mammalian spinal networks.Sci Rep. 2020 Oct 2;10(1):16429. doi: 10.1038/s41598-020-73230-w. Sci Rep. 2020. PMID: 33009442 Free PMC article.
-
Serotonergic modulation of post-synaptic inhibition and locomotor alternating pattern in the spinal cord.Front Neural Circuits. 2014 Aug 28;8:102. doi: 10.3389/fncir.2014.00102. eCollection 2014. Front Neural Circuits. 2014. PMID: 25221477 Free PMC article. Review.
-
Electrical stimulation affects the differentiation of transplanted regionally specific human spinal neural progenitor cells (sNPCs) after chronic spinal cord injury.Stem Cell Res Ther. 2023 Dec 20;14(1):378. doi: 10.1186/s13287-023-03597-w. Stem Cell Res Ther. 2023. PMID: 38124191 Free PMC article.
-
Reversible disorganization of the locomotor pattern after neonatal spinal cord transection in the rat.J Neurosci. 2003 Mar 1;23(5):1924-32. doi: 10.1523/JNEUROSCI.23-05-01924.2003. J Neurosci. 2003. PMID: 12629197 Free PMC article.
-
The role of the serotonergic system in locomotor recovery after spinal cord injury.Front Neural Circuits. 2015 Feb 9;8:151. doi: 10.3389/fncir.2014.00151. eCollection 2014. Front Neural Circuits. 2015. PMID: 25709569 Free PMC article. Review.
References
-
- Ben-Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24:353–360. - PubMed
-
- Berger AJ, Bayliss DA, Viana F. Development of hypoglossal motoneurons. J Appl Physiol. 1996;81:1039–1048. - PubMed
-
- Bolles RC, Woods PJ. The ontogeny of behaviour in the albino rat. Anim Behav. 1964;12:427–441.
-
- Bregman BS. Development of serotonin immunoreactivity in the rat spinal cord and its plasticity after neonatal spinal cord lesions. Brain Res. 1987;431:245–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
