Dopamine modulates excitability of spiny neurons in the avian basal ganglia
- PMID: 12077216
- PMCID: PMC6757730
- DOI: 10.1523/JNEUROSCI.22-12-05210.2002
Dopamine modulates excitability of spiny neurons in the avian basal ganglia
Erratum in
- J Neurosci 2002 Aug 1;22(15):6835
Abstract
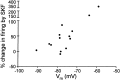
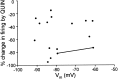
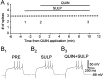
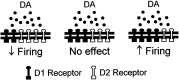
The neural substrate of vocal learning in songbirds is an accessible system for studying motor learning and motor control in vertebrates. In the so-called song system, the anterior forebrain pathway (AFP), which is essential for song learning, resembles the mammalian basal ganglia-thalamocortical loop in its macroscopic organization, neuronal intrinsic properties, and microcircuitry. Area X, the first station in the AFP, and the surrounding lobus parolfactorius (LPO), are both parts of the avian basal ganglia. Like their mammalian counterparts, they receive dense dopaminergic innervation from the midbrain, but the physiological functions of this projection remain unclear. In this study, we investigated the effect of dopamine (DA) on excitability of spiny neurons in area X and LPO. We recorded from neurons in brain slices of adult zebra finches and Bengalese finches, using whole-cell and perforated-patch recording techniques in current-clamp configuration. We found that DA modulates excitability in spiny neurons; activation of D1- and D2-like DA receptors enhances and reduces excitability, respectively. These effects are similar to those observed in the mammalian neostriatum, with the main difference being that D1-like DA receptor activation enhances excitability in avian spiny neurons at hyperpolarized states. Our findings also indicate that some spiny neurons express both receptor types and suggest that receptor colocalization in the entire population can account for the spectrum of DA actions. The diversity of DA actions enables the DA system to fine-tune the dynamics of the song system and allows flexible control over song learning and production.
Figures







Similar articles
-
Presynaptic depression of glutamatergic synaptic transmission by D1-like dopamine receptor activation in the avian basal ganglia.J Neurosci. 2003 Jul 9;23(14):6086-95. doi: 10.1523/JNEUROSCI.23-14-06086.2003. J Neurosci. 2003. PMID: 12853427 Free PMC article.
-
Striatal dopamine modulates basal ganglia output and regulates social context-dependent behavioral variability through D1 receptors.J Neurosci. 2010 Apr 21;30(16):5730-43. doi: 10.1523/JNEUROSCI.5974-09.2010. J Neurosci. 2010. PMID: 20410125 Free PMC article.
-
Intrinsic and synaptic properties of neurons in an avian thalamic nucleus during song learning.J Neurophysiol. 2002 Oct;88(4):1903-14. doi: 10.1152/jn.2002.88.4.1903. J Neurophysiol. 2002. PMID: 12364516
-
Electrophysiological analysis of a songbird basal ganglia circuit essential for vocal plasticity.Brain Res Bull. 2002 Feb-Mar 1;57(3-4):529-32. doi: 10.1016/s0361-9230(01)00690-6. Brain Res Bull. 2002. PMID: 11923022 Review.
-
Social modulation of learned behavior by dopamine in the basal ganglia: insights from songbirds.J Physiol Paris. 2013 Jun;107(3):219-29. doi: 10.1016/j.jphysparis.2012.09.002. Epub 2012 Sep 29. J Physiol Paris. 2013. PMID: 23032272 Review.
Cited by
-
Dopamine activates noradrenergic receptors in the preoptic area.J Neurosci. 2002 Nov 1;22(21):9320-30. doi: 10.1523/JNEUROSCI.22-21-09320.2002. J Neurosci. 2002. PMID: 12417657 Free PMC article.
-
Context-dependent relationships between locus coeruleus firing patterns and coordinated neural activity in the anterior cingulate cortex.Elife. 2022 Jan 7;11:e63490. doi: 10.7554/eLife.63490. Elife. 2022. PMID: 34994344 Free PMC article.
-
Neurons in a forebrain nucleus required for vocal plasticity rapidly switch between precise firing and variable bursting depending on social context.J Neurosci. 2008 Dec 3;28(49):13232-47. doi: 10.1523/JNEUROSCI.2250-08.2008. J Neurosci. 2008. PMID: 19052215 Free PMC article.
-
Plasticity in D1-like receptor expression is associated with different components of cognitive processes.PLoS One. 2012;7(5):e36484. doi: 10.1371/journal.pone.0036484. Epub 2012 May 4. PLoS One. 2012. PMID: 22574169 Free PMC article.
-
Differential relationships between D1 and D2 dopamine receptor expression in the medial preoptic nucleus and sexually-motivated song in male European starlings (Sturnus vulgaris).Neuroscience. 2015 Aug 20;301:289-97. doi: 10.1016/j.neuroscience.2015.06.011. Epub 2015 Jun 12. Neuroscience. 2015. PMID: 26079111 Free PMC article.
References
-
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3:226–230. - PubMed
-
- Blanton MG, Lo Turco JJ, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989;30:203–210. - PubMed
-
- Bottjer SW. The distribution of tyrosine-hydroxylase immunoreactivity in the brains of male and female zebra finches. J Neurobiol. 1993;24:51–69. - PubMed
-
- Bottjer SW, Johnson F. Circuits, hormones, and learning: vocal behavior in songbirds. J Neurobiol. 1997;33:602–618. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
