A possible evolutionary origin for the Mn4 cluster of the photosynthetic water oxidation complex from natural MnO2 precipitates in the early ocean
- PMID: 12077302
- PMCID: PMC124339
- DOI: 10.1073/pnas.132266199
A possible evolutionary origin for the Mn4 cluster of the photosynthetic water oxidation complex from natural MnO2 precipitates in the early ocean
Abstract
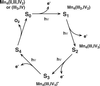
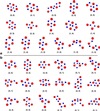
The photosynthetic water oxidation complex consists of a cluster of four Mn atoms bridged by O atoms, associated with Ca2+ and Cl-, and incorporated into protein. The structure is similar in higher plants and algae, as well as in cyanobacteria of more ancient lineage, dating back more than 2.5 billion years ago on Earth. It has been proposed that the proto-enzyme derived from a component of a natural early marine manganese precipitate that contained a CaMn4O9 cluster. A variety of MnO2 minerals are found in nature. Three major classes are spinels, sheet-like layered structures, and three-dimensional networks that contain parallel tunnels. These relatively open structures readily incorporate cations (Na+, Li+, Mg2+, Ca2+, Ba2+, H+, and even Mn2+) and water. The minerals have different ratios of Mn(III) and Mn(IV) octahedrally coordinated to oxygens. Using x-ray spectroscopy we compare the chemical structures of Mn in the minerals with what is known about the arrangement in the water oxidation complex to define the parameters of a structural model for the photosynthetic catalytic site. This comparison provides for the structural model a set of candidate Mn(4) clusters-some previously proposed and considered and others entirely novel.
Figures


Similar articles
-
Nano-sized layered Mn oxides as promising and biomimetic water oxidizing catalysts for water splitting in artificial photosynthetic systems.J Photochem Photobiol B. 2014 Apr 5;133:124-39. doi: 10.1016/j.jphotobiol.2014.03.005. Epub 2014 Mar 19. J Photochem Photobiol B. 2014. PMID: 24727405 Review.
-
Photochemical water oxidation by crystalline polymorphs of manganese oxides: structural requirements for catalysis.J Am Chem Soc. 2013 Mar 6;135(9):3494-501. doi: 10.1021/ja310286h. Epub 2013 Feb 25. J Am Chem Soc. 2013. PMID: 23391134
-
Photosynthetic water oxidation: insights from manganese model chemistry.Acc Chem Res. 2015 Mar 17;48(3):567-74. doi: 10.1021/ar5004175. Epub 2015 Mar 2. Acc Chem Res. 2015. PMID: 25730258
-
Development of bioinspired Mn4O4-cubane water oxidation catalysts: lessons from photosynthesis.Acc Chem Res. 2009 Dec 21;42(12):1935-43. doi: 10.1021/ar900249x. Acc Chem Res. 2009. PMID: 19908827
-
An evaluation of structural models for the photosynthetic water-oxidizing complex derived from spectroscopic and X-ray diffraction signatures.J Biol Inorg Chem. 2002 Jan;7(1-2):2-22. doi: 10.1007/s00775-001-0305-3. Epub 2001 Nov 8. J Biol Inorg Chem. 2002. PMID: 11862536 Review.
Cited by
-
Photoelectric conversion on Earth's surface via widespread Fe- and Mn-mineral coatings.Proc Natl Acad Sci U S A. 2019 May 14;116(20):9741-9746. doi: 10.1073/pnas.1902473116. Epub 2019 Apr 22. Proc Natl Acad Sci U S A. 2019. PMID: 31010932 Free PMC article.
-
Theoretical elucidation of the structure, bonding, and reactivity of the CaMn4Ox clusters in the whole Kok cycle for water oxidation embedded in the oxygen evolving center of photosystem II. New molecular and quantum insights into the mechanism of the O-O bond formation.Photosynth Res. 2024 Dec;162(2-3):291-330. doi: 10.1007/s11120-023-01053-7. Epub 2023 Nov 9. Photosynth Res. 2024. PMID: 37945776 Free PMC article. Review.
-
X-ray spectroscopy of the Mn4Ca cluster in the water-oxidation complex of Photosystem II.Photosynth Res. 2005;85(1):73-86. doi: 10.1007/s11120-005-0638-9. Photosynth Res. 2005. PMID: 15977060 Free PMC article. Review.
-
Mn4Ca cluster in photosynthesis: where and how water is oxidized to dioxygen.Chem Rev. 2014 Apr 23;114(8):4175-205. doi: 10.1021/cr4004874. Epub 2014 Mar 31. Chem Rev. 2014. PMID: 24684576 Free PMC article. Review. No abstract available.
-
Tribute to Kenneth Sauer (1931-2022): a mentor, a role-model, and an inspiration to all in the field of photosynthesis.Photosynth Res. 2024 Dec;162(2-3):103-138. doi: 10.1007/s11120-024-01119-0. Epub 2024 Nov 13. Photosynth Res. 2024. PMID: 39535662 Free PMC article.
References
-
- Lowe D R. In: Early Life on Earth, Nobel Symposium No. 84. Bengtson S, editor. New York: Columbia Univ. Press; 1994. pp. 24–35.
-
- Kasting J F. Science. 1993;259:920–926. - PubMed
-
- Towe K M. In: Early Life on Earth, Nobel Symposium No. 84. Bengtson S, editor. New York: Columbia Univ. Press; 1994. pp. 36–47.
-
- Chang S. In: Early Life on Earth, Nobel Symposium No. 84. Bengtson S, editor. New York: Columbia Univ. Press; 1994. pp. 10–23.
-
- Schopf J W, Klein C, editors. The Proterozoic Biosphere. Cambridge, U.K.: Cambridge Univ. Press; 1992.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

