Identification of a gene encoding MGAT1, a monoacylglycerol acyltransferase
- PMID: 12077311
- PMCID: PMC124292
- DOI: 10.1073/pnas.132274899
Identification of a gene encoding MGAT1, a monoacylglycerol acyltransferase
Abstract
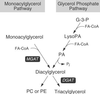
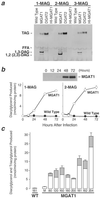
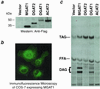
Acyl-CoA:monoacylglycerol acyltransferase (MGAT) catalyzes the synthesis of diacylglycerol, the precursor of physiologically important lipids such as triacylglycerol and phospholipids. In the intestine, MGAT plays a major role in the absorption of dietary fat because resynthesis of triacylglycerol is required for the assembly of lipoproteins that transport absorbed fat to other tissues. MGAT activity has also been reported in mammalian liver and white adipose tissue. However, MGAT has never been purified to homogeneity from mammalian tissues, and its gene has not been cloned. We identified a gene that encodes an MGAT (MGAT1) in mice. This gene has sequence homology with members of a recently identified diacylglycerol acyltransferase gene family. Expression of the MGAT1 cDNA in insect cells markedly increased MGAT activity in cell membranes. In addition, MGAT activity was proportional to the level of MGAT1 protein expressed, and the amount of diacylglycerol produced depended on the concentration of either of its substrates, oleoyl-CoA or monooleoylglycerol. In mice, MGAT1 expression and MGAT activity were detected in the stomach, kidney, white and brown adipose tissue, and liver. However, MGAT1 was not expressed in the small intestine, implying the existence of a second MGAT gene. The identification of the MGAT1 gene should greatly facilitate research on the identification of the intestinal MGAT gene and on the function of MGAT enzymes in mammalian glycerolipid metabolism.
Figures



References
-
- Ron D, Kazanietz M G. FASEB J. 1999;13:1658–1676. - PubMed
-
- Lehner R, Kuksis A. Prog Lipid Res. 1996;35:169–201. - PubMed
-
- Bell R M, Coleman R A. Annu Rev Biochem. 1980;49:459–487. - PubMed
-
- Coleman R A, Haynes E B. J Biol Chem. 1984;259:8934–8938. - PubMed
-
- Mostafa N, Bhat B G, Coleman R A. Lipids. 1994;29:785–791. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

