Overexpression, purification, and site-directed spin labeling of the Nramp metal transporter from Mycobacterium leprae
- PMID: 12077319
- PMCID: PMC124330
- DOI: 10.1073/pnas.142287699
Overexpression, purification, and site-directed spin labeling of the Nramp metal transporter from Mycobacterium leprae
Erratum in
- Proc Natl Acad Sci U S A. 2003 Jan 21;100(2):763.. Hummell David [corrected to Hummel David]
Abstract
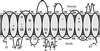
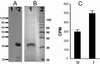
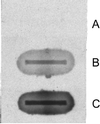
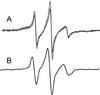
It has long been recognized that the pathogenicity of a broad range of intracellular parasites depends on the availability of transition metal ions, especially iron. Nramp1 (natural resistance-associated macrophage protein 1), a proton-coupled divalent metal ion transporter, has been identified as a controlling factor in the resistance or susceptibility to infection with a diverse range of intracellular pathogens such as Toxoplasma, Salmonella, Mycobacterium, and Leishmania. The role of divalent metal ion transport is even more compelling given the existence of Nramp homologs in several intracellular parasites, such as mycobacteria. We have confirmed the functional homology of the Nramp homologue from Mycobacterium leprae by using a yeast complementation assay for divalent cation uptake. To facilitate a concerted biochemical and structural analysis of this important class of transporters, the M. leprae Nramp was expressed in Escherichia coli. Dual affinity tags were engineered at the N and C termini to allow for isolation of full-length protein at >95% purity. Site-directed spin labeling of Cys-299 reveals a flexible hinge-like domain. A weak dipolar interaction is detected between the nitroxide and paramagnetic transition ions, indicating this position is approximately 19 A from the nearest high affinity binding site.
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

