Kinetic analysis of estrogen receptor/ligand interactions
- PMID: 12077320
- PMCID: PMC124311
- DOI: 10.1073/pnas.142288199
Kinetic analysis of estrogen receptor/ligand interactions
Abstract
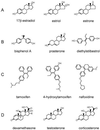
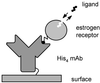
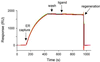
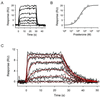
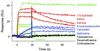
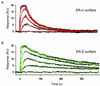
Surface plasmon resonance biosensor technology was used to directly measure the binding interactions of small molecules to the ligand-binding domain of human estrogen receptor. In a screening mode, specific ligands of the receptor were easily discerned from nonligands. In a high-resolution mode, the association and dissociation phase binding responses were shown to be reproducible and could be fit globally to a simple interaction model to extract reaction rate constants. On average, antagonist ligands (such as tamoxifen and nafoxidine) were observed to bind to the receptor with association rates that were 500-fold slower than agonists (such as estriol and beta-estradiol). This finding is consistent with these antagonists binding to an altered conformation of the receptor. The biosensor assay also could identify subtle differences in how the same ligand interacted with two different isoforms of the receptor (alpha and beta). The biosensor's ability to determine kinetic rate constants for small molecule/protein interactions provides unique opportunities to understand the mechanisms associated with complex formation as well as new information to drive the optimization of drug candidates.
Figures

References
-
- Rich R L, Day Y S, Morton T A, Myszka D G. Anal Biochem. 2001;296:197–207. - PubMed
-
- Myszka D G, Rich R L. Pharm Sci Technol Today. 2000;3:310–317. - PubMed
-
- Rich R L, Myszka D G. Curr Opin Biotechnol. 2000;11:54–61. - PubMed
-
- Gao H, Williams C, Labute P, Bajorath J. J Chem Inf Comput Sci. 1999;39:164–168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

