Cellophane based mini-prep method for DNA extraction from the filamentous fungus Trichoderma reesei
- PMID: 12079503
- PMCID: PMC116681
- DOI: 10.1186/1471-2180-2-14
Cellophane based mini-prep method for DNA extraction from the filamentous fungus Trichoderma reesei
Abstract
Background: Methods for the extraction of DNA from filamentous fungi are frequently laborious and time consuming because most of the available protocols include maceration in liquid nitrogen after the mycelium has been grown in a liquid culture. This paper describes a new method to replace those steps, which involves the growth of the mycelium on cellophane disks overlaid on solid medium and the use of glass beads for cell wall disruption.
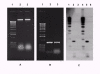
Results: Extractions carried out by this method provided approximately 2 microg of total DNA per cellophane disk for the filamentous fungus Trichoderma reesei. To assess the DNA's quality, we made a PCR (Polymerase Chain Reaction) amplification of a gene introduced by a transformation in this fungus's genome (hph gene), with successful results. We also confirmed the quality of the DNA by the use of Southern blotting to analyze the presence of the same gene, which was easily detected, resulting in a sharply defined and strong band.
Conclusions: The use of this method enabled us to obtain pure DNA from Trichoderma reesei, dispensing with the laborious and time-consuming steps involved in most protocols. The DNA obtained was found to be suitable for PCR and Southern blot analyses. Another advantage of this method is the fact that several samples can be processed simultaneously, growing the fungus on multiple well cell culture plates. In addition, the absence of maceration also reduces sample handling, minimizing the risks of contamination, a particularly important factor in work involving PCR.
Figures
Similar articles
-
Biolistic transformation of Trichoderma reesei using the Bio-Rad seven barrels Hepta Adaptor system.J Microbiol Methods. 2002 Nov;51(3):393-9. doi: 10.1016/s0167-7012(02)00126-4. J Microbiol Methods. 2002. PMID: 12223300
-
A rapid and inexpensive method for isolation of total DNA from Trichoderma spp (Hypocreaceae).Genet Mol Res. 2012 May 15;11(2):1379-84. doi: 10.4238/2012.May.15.8. Genet Mol Res. 2012. PMID: 22653584
-
Transcriptional monitoring of steady state and effects of anaerobic phases in chemostat cultures of the filamentous fungus Trichoderma reesei.BMC Genomics. 2006 Oct 2;7:247. doi: 10.1186/1471-2164-7-247. BMC Genomics. 2006. PMID: 17010217 Free PMC article.
-
Deciphering the molecular mechanisms behind cellulase production in Trichoderma reesei, the hyper-cellulolytic filamentous fungus.Biosci Biotechnol Biochem. 2016 Sep;80(9):1712-29. doi: 10.1080/09168451.2016.1171701. Epub 2016 Apr 14. Biosci Biotechnol Biochem. 2016. PMID: 27075508 Review.
-
Production of recombinant proteins in the filamentous fungus Trichoderma reesei.Curr Opin Biotechnol. 1995 Oct;6(5):534-7. doi: 10.1016/0958-1669(95)80088-3. Curr Opin Biotechnol. 1995. PMID: 7579664 Review.
Cited by
-
Reconstructing NOD-like receptor alleles with high internal conservation in Podospora anserina using long-read sequencing.Microb Genom. 2025 Jul;11(7):001442. doi: 10.1099/mgen.0.001442. Microb Genom. 2025. PMID: 40601474 Free PMC article.
-
Physiological Responses of Aspergillus niger Challenged with Itraconazole.Antimicrob Agents Chemother. 2021 May 18;65(6):e02549-20. doi: 10.1128/AAC.02549-20. Print 2021 May 18. Antimicrob Agents Chemother. 2021. PMID: 33820768 Free PMC article.
-
Identification and characterization of dermatophyte species and strains with PCR amplification.Exp Ther Med. 2014 Aug;8(2):545-550. doi: 10.3892/etm.2014.1785. Epub 2014 Jun 13. Exp Ther Med. 2014. PMID: 25009617 Free PMC article.
-
Volatile Compound-Mediated Recognition and Inhibition Between Trichoderma Biocontrol Agents and Fusarium oxysporum.Front Microbiol. 2018 Oct 31;9:2614. doi: 10.3389/fmicb.2018.02614. eCollection 2018. Front Microbiol. 2018. PMID: 30455673 Free PMC article.
-
Genome sequencing and de novo assembly of Trichoderma longibrachiatum isolate collected from Florida agricultural soils.Microbiol Resour Announc. 2024 Jan 17;13(1):e0090623. doi: 10.1128/mra.00906-23. Epub 2023 Dec 11. Microbiol Resour Announc. 2024. PMID: 38078731 Free PMC article.
References
-
- Specht CA, DiRusso CC, Novotny CP, Ullrich RC. A method for extracting high-molecular-weight deoxyribonucleic acid from fungi. Anal Biochem. 1982;119:158–163. - PubMed
-
- de Graaff L, van den BH, Visser J. Isolation and transformation of the pyruvate kinase gene of Aspergillus nidulans. Curr Genet. 1988;13:315–321. - PubMed
-
- Challen MP, Moore AJ, Martinez-Carrera D. Facile extraction and purification of filamentous fungal DNA. Biotechniques. 1995;18:975–978. - PubMed
-
- Raeder U, Broda P. Rapid preparation of DNA from filamentous fungi. Lett Appl Microbiol. 1985. pp. 17–20.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

