MAP2 and tau bind longitudinally along the outer ridges of microtubule protofilaments
- PMID: 12082079
- PMCID: PMC2173547
- DOI: 10.1083/jcb.200201048
MAP2 and tau bind longitudinally along the outer ridges of microtubule protofilaments
Abstract
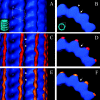
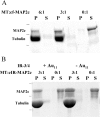
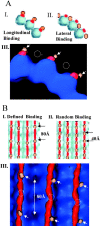
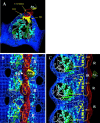
MAP2 and tau exhibit microtubule-stabilizing activities that are implicated in the development and maintenance of neuronal axons and dendrites. The proteins share a homologous COOH-terminal domain, composed of three or four microtubule binding repeats separated by inter-repeats (IRs). To investigate how MAP2 and tau stabilize microtubules, we calculated 3D maps of microtubules fully decorated with MAP2c or tau using cryo-EM and helical image analysis. Comparing these maps with an undecorated microtubule map revealed additional densities along protofilament ridges on the microtubule exterior, indicating that MAP2c and tau form an ordered structure when they bind microtubules. Localization of undecagold attached to the second IR of MAP2c showed that IRs also lie along the ridges, not between protofilaments. The densities attributable to the microtubule-associated proteins lie in close proximity to helices 11 and 12 and the COOH terminus of tubulin. Our data further suggest that the evolutionarily maintained differences observed in the repeat domain may be important for the specific targeting of different repeats to either alpha or beta tubulin. These results provide strong evidence suggesting that MAP2c and tau stabilize microtubules by binding along individual protofilaments, possibly by bridging the tubulin interfaces.
Figures



References
-
- Aiyar, A. 2000. The use of CLUSTAL W and CLUSTAL X for multiple sequence alignment. Methods Mol. Biol. 132:221–241. - PubMed
-
- Carragher, B.O., M. Whittaker, and R.A. Milligan. 1996. Helical processing using PHOELIX. J. Struct. Biol. 116:107–112. - PubMed
-
- Chau, M.F., M.J. Radeke, C. de Ines, I. Barasoain, L.A. Kohlstaedt, and S.C. Feinstein. 1998. The microtubule-associated protein tau cross-links to two distinct sites on each alpha and beta tubulin monomer via separate domains. Biochemistry. 37:17692–17703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

