Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization
- PMID: 12082181
- PMCID: PMC123160
- DOI: 10.1073/pnas.142063399
Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization
Abstract
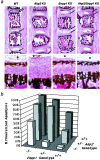
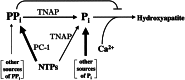
Osteoblasts mineralize bone matrix by promoting hydroxyapatite crystal formation and growth in the interior of membrane-limited matrix vesicles (MVs) and by propagating the crystals onto the collagenous extracellular matrix. Two osteoblast proteins, tissue-nonspecific alkaline phosphatase (TNAP) and plasma cell membrane glycoprotein-1 (PC-1) are involved in this process. Mutations in the TNAP gene result in the inborn error of metabolism known as hypophosphatasia, characterized by poorly mineralized bones, spontaneous fractures, and elevated extracellular concentrations of inorganic pyrophosphate (PP(i)). PP(i) suppresses the formation and growth of hydroxyapatite crystals. PP(i) is produced by the nucleoside triphosphate pyrophosphohydrolase activity of a family of isozymes, with PC-1 being the only member present in MVs. Mice with spontaneous mutations in the PC-1 gene have hypermineralization abnormalities that include osteoarthritis and ossification of the posterior longitudinal ligament of the spine. Here, we show the respective correction of bone mineralization abnormalities in knockout mice null for both the TNAP (Akp2) and PC-1 (Enpp1) genes. Each allele of Akp2 and Enpp1 has a measurable influence on mineralization status in vivo. Ex vivo experiments using cultured double-knockout osteoblasts and their MVs demonstrate normalization of PP(i) content and mineral deposition. Our data provide evidence that TNAP and PC-1 are key regulators of the extracellular PP(i) concentrations required for controlled bone mineralization. Our results suggest that inhibiting PC-1 function may be a viable therapeutic strategy for hypophosphatasia. Conversely, interfering with TNAP activity may correct pathological hyperossification because of PP(i) insufficiency.
Figures


References
-
- Anderson H C. Clin Orthop Relat Res. 1995;314:266–280. - PubMed
-
- Boskey A L. Connect Tissue Res. 1996;35:357–363. - PubMed
-
- Henthorn P, Millán J L, Leboy P. In: Principles of Bone Biology. Seibel M J, Robins S P, Bilezikian J P, editors. San Diego: Academic; 1999. pp. 127–136.
-
- Henthorn P S, Whyte M P. Clin Chem. 1992;38:2501–2505. - PubMed
-
- Whyte M P. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, Childs B, Kinzler K W, Vogelstein B, editors. New York: McGraw–Hill; 2001. pp. 5313–5329.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

