Interactions of flavones and other phytochemicals with adenosine receptors
- PMID: 12083460
- PMCID: PMC3429336
- DOI: 10.1007/978-1-4757-5235-9_15
Interactions of flavones and other phytochemicals with adenosine receptors
Abstract
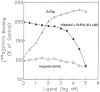
Dietary flavonoids have varied effects on animal cells, such as inhibition of platelet binding and aggregation, inhibition of inflammation, and anticancer properties, but the mechanisms of these effects remain largely unexplained. Adenosine receptors are involved in the homeostasis of the immune, cardiovascular, and central nervous systems, and adenosine agonists/antagonists exert many similar effects. The affinity of flavonoids and other phytochemicals to adenosine receptors suggests that a wide range of natural substances in the diet may potentially block the effects of endogenous adenosine. We used competitive radioligand binding assays to screen flavonoid libraries for affinity and a computational CoMFA analysis of flavonoids to compare steric and electrostatic requirements for ligand recognition at three subtypes of adenosine receptors. Flavone derivatives, such as galangin, were found to bind to three subtypes of adenosine receptors in the microM range. Pentamethylmorin (Ki 2.65 microM) was 14- to 17-fold selective for human A3 receptors than for A1 and A2A receptors. An isoflavone, genistein, was found to bind to A1 receptors. Aurones, such as hispidol (Ki 350 nM) are selective A1 receptor antagonists, and, like genistein, are present in soy. The flavones, chemically optimized for receptor binding, have led to the antagonist, MRS 1067 (3,6-dichloro-2'-(isopropoxy)4'-methylflavone), which is 200-fold more selective for human A3 than A1 receptors. Adenosine receptor antagonism, therefore, may be important in the spectrum of biological activities reported for the flavonoids.
Figures
Similar articles
-
Synthesis and biological activities of flavonoid derivatives as A3 adenosine receptor antagonists.J Med Chem. 1996 Jun 7;39(12):2293-301. doi: 10.1021/jm950923i. J Med Chem. 1996. PMID: 8691424 Free PMC article.
-
Interactions of flavonoids and other phytochemicals with adenosine receptors.J Med Chem. 1996 Feb 2;39(3):781-8. doi: 10.1021/jm950661k. J Med Chem. 1996. PMID: 8576921 Free PMC article.
-
Flavonoid derivatives as adenosine receptor antagonists: a comparison of the hypothetical receptor binding site based on a comparative molecular field analysis model.J Med Chem. 1998 Jan 1;41(1):46-52. doi: 10.1021/jm970446z. J Med Chem. 1998. PMID: 9438021 Free PMC article.
-
Adenosine A3 receptors: novel ligands and paradoxical effects.Trends Pharmacol Sci. 1998 May;19(5):184-91. doi: 10.1016/s0165-6147(98)01203-6. Trends Pharmacol Sci. 1998. PMID: 9652191 Free PMC article. Review.
-
Purinergic signalling in brain ischemia.Neuropharmacology. 2016 May;104:105-30. doi: 10.1016/j.neuropharm.2015.11.007. Epub 2015 Nov 12. Neuropharmacology. 2016. PMID: 26581499 Review.
Cited by
-
Possible Combinatorial Utilization of Phytochemicals and Extracellular Vesicles for Wound Healing and Regeneration.Int J Mol Sci. 2024 Sep 26;25(19):10353. doi: 10.3390/ijms251910353. Int J Mol Sci. 2024. PMID: 39408681 Free PMC article. Review.
-
Evaluation of Diuretic Activity and Phytochemical Contents of Aqueous Extract of the Shoot Apex of Podocarpus falcactus.J Exp Pharmacol. 2020 Dec 16;12:629-641. doi: 10.2147/JEP.S287277. eCollection 2020. J Exp Pharmacol. 2020. PMID: 33364857 Free PMC article.
-
Anticoagulant effect and safety assessment of an aqueous extract of Pseudocedrela kotschyi (Schweinf.) harms and Adenia cissampeloides (Planch. Ex Hook.) harms.J Intercult Ethnopharmacol. 2016 Mar 30;5(2):153-61. doi: 10.5455/jice.20160324054355. eCollection 2016 Mar-Apr. J Intercult Ethnopharmacol. 2016. PMID: 27104036 Free PMC article.
-
Methoxy substituted 2-benzylidene-1-indanone derivatives as A1 and/or A2A AR antagonists for the potential treatment of neurological conditions.Medchemcomm. 2019 Jan 8;10(2):300-309. doi: 10.1039/c8md00540k. eCollection 2019 Feb 1. Medchemcomm. 2019. PMID: 30881617 Free PMC article.
-
A Potential Alternative against Neurodegenerative Diseases: Phytodrugs.Oxid Med Cell Longev. 2016;2016:8378613. doi: 10.1155/2016/8378613. Epub 2016 Jan 6. Oxid Med Cell Longev. 2016. PMID: 26881043 Free PMC article. Review.
References
-
- Ares JJ, Pong SF, Outt PE, Blank MA, Murray PD, Portlock DE. The binding of flavonoids to adenosine receptors; ACS 209th National Meeting; Chicago, IL. April 1995; 1995. Abstract MEDI 190.
-
- Barnes S, Kim H, Darley-Usmar V, Patel R, Xu J, Boersma B, Luo M. Beyond ERa and ERb: Estrogen receptor binding is only part of the isoflavone story. J. Nutr. 2000;130:656S–657S. - PubMed
-
- Beaven MA, Ramkumar V, Ali H. Adenosine A, receptors in mast cells. Trends Pharmacol. Sci. 1994;15:13–14. - PubMed
-
- Beretz A, Cazenave JP. The effect of flavonoids on blood-vessel wall interactions. In: Cody V, Middleton E Jr., Harborne, Beretz JB, editors. Plant Flavonoids in Biology and Medicine II: Biochemical. Cellular, and Medicinal Properties. Alan R. Liss; New York: 1988. pp. 187–200. A. - PubMed
-
- Coward L, Barnes N, Setchell K, Barnes S. Genistein, daidzein, and their B-glycoside conjugates: antitumor isoflavones in soybean foods from American and Asian diets. J. Agric. Food Chem. 1993;41:1961–1967.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

