Current-dependent block of rabbit sino-atrial node I(f) channels by ivabradine
- PMID: 12084770
- PMCID: PMC2238187
- DOI: 10.1085/jgp.20028593
Current-dependent block of rabbit sino-atrial node I(f) channels by ivabradine
Abstract
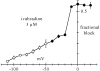
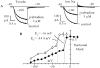
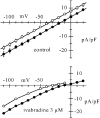
"Funny" (f-) channels have a key role in generation of spontaneous activity of pacemaker cells and mediate autonomic control of cardiac rate; f-channels and the related neuronal h-channels are composed of hyperpolarization-activated, cyclic nucleotide-gated (HCN) channel subunits. We have investigated the block of f-channels of rabbit cardiac sino-atrial node cells by ivabradine, a novel heart rate-reducing agent. Ivabradine is an open-channel blocker; however, block is exerted preferentially when channels deactivate on depolarization, and is relieved by long hyperpolarizing steps. These features give rise to use-dependent behavior. In this, the action of ivabradine on f-channels is similar to that reported of other rate-reducing agents such as UL-FS49 and ZD7288. However, other features of ivabradine-induced block are peculiar and do not comply with the hypothesis that the voltage-dependence of block is entirely attributable to either the sensitivity of ivabradine-charged molecules to the electrical field in the channel pore, or to differential affinity to different channel states, as has been proposed for UL-FS49 (DiFrancesco, D. 1994. Pflugers Arch. 427:64-70) and ZD7288 (Shin, S.K., B.S. Rotheberg, and G. Yellen. 2001. J. Gen. Physiol. 117:91-101), respectively. Experiments where current flows through channels is modified without changing membrane voltage reveal that the ivabradine block depends on the current driving force, rather than voltage alone, a feature typical of block induced in inwardly rectifying K(+) channels by intracellular cations. Bound drug molecules do not detach from the binding site in the absence of inward current through channels, even if channels are open and the drug is therefore not "trapped" by closed gates. Our data suggest that permeation through f-channel pores occurs according to a multiion, single-file mechanism, and that block/unblock by ivabradine is coupled to ionic flow. The use-dependence resulting from specific features of I(f) block by ivabradine amplifies its rate-reducing ability at high spontaneous rates and may be useful to clinical applications.
Figures





References
-
- Beaumont, V., and R.S. Zucker. 2000. Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nat. Neurosci. 3:133–141. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

