cAMP increases density of ENaC subunits in the apical membrane of MDCK cells in direct proportion to amiloride-sensitive Na(+) transport
- PMID: 12084777
- PMCID: PMC2311399
- DOI: 10.1085/jgp.20018547
cAMP increases density of ENaC subunits in the apical membrane of MDCK cells in direct proportion to amiloride-sensitive Na(+) transport
Abstract
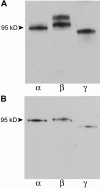
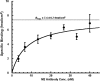
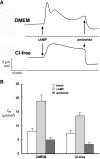
Antidiuretic hormone and/or cAMP increase Na(+) transport in the rat renal collecting duct and similar epithelia, including Madin-Darby canine kidney (MDCK) cell monolayers grown in culture. This study was undertaken to determine if that increment in Na(+) transport could be explained quantitatively by an increased density of ENaC Na(+) channels in the apical membrane. MDCK cells with no endogenous ENaC expression were retrovirally transfected with rat alpha-, beta-, and gammaENaC subunits, each of which were labeled with the FLAG epitope in their extracellular loop as described previously (Firsov, D., L. Schild, I. Gautschi, A.-M. Mérillat, E. Schneeberger, and B.C. Rossier. 1996. PROC: Natl. Acad. Sci. USA. 93:15370-15375). The density of ENaC subunits was quantified by specific binding of (125)I-labeled anti-FLAG antibody (M2) to the apical membrane, which was found to be a saturable function of M2 concentration with half-maximal binding at 4-8 nM. Transepithelial Na(+) transport was measured as the amiloride-sensitive short-circuit current (AS-I(sc)) across MDCK cells grown on permeable supports. Specific M2 binding was positively correlated with AS-I(sc) measured in the same experiments. Stimulation with cAMP (20 microM 8-p-chlorothio-cAMP plus 200 microM IBMX) significantly increased AS-I(sc) from 11.2 +/- 1.3 to 18.1 +/- 1.3 microA/cm(2). M2 binding (at 1.7 nM M2) increased in direct proportion to AS-I(sc) from 0.62 +/- 0.13 to 1.16 +/- 0.18 fmol/cm(2). Based on the concentration dependence of M2 binding, the quantity of Na(+) channels per unit of AS-I(sc) was calculated to be the same in the presence and absence of cAMP, 0.23 +/- 0.04 and 0.21 +/-0.05 fmol/microA, respectively. These values would be consistent with a single channel conductance of approximately 5 pS (typically reported for ENaC channels) only if the open probability is <0.02, i.e., less than one-tenth of the typical value. We interpret the proportional increases in binding and AS-I(sc) to indicate that the increased density of ENaC subunits in the apical membrane can account completely for the I(sc) increase produced by cAMP.
Figures




References
-
- Berdiev, B.K., A.G. Prat, H.F. Cantiello, D.A. Ausiello, C.M. Fuller, B. Jovov, D.J. Benos, and I.I. Ismailov. 1996. Regulation of epithelial sodium channels by short actin filaments. J. Biol. Chem. 271:17704–17710. - PubMed
-
- Bindels, R.J.M., J.A. Schafer, and M.C. Reif. 1988. Stimulation of sodium transport by aldosterone and arginine vasotocin in A6 cells. Biochim. Biophys. Acta. 972:320–330. - PubMed
-
- Blazer-Yost, B.L., and S.I. Helman. 1997. The amiloride-sensitive epithelial Na+ channel: binding sites and channel densities. Am. J. Physiol. 272:C761–C769. - PubMed
-
- Bradford, A.L., I.I. Ismailov, J.M. Achard, D.G. Warnock, J.K. Bubien, and D.J. Benos. 1995. Immunopurification and functional reconstitution of a Na+ channel complex from rat lymphocytes. Am. J. Physiol. 269:C601–C611. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

