Interactions between Eph kinases and ephrins provide a mechanism to support platelet aggregation once cell-to-cell contact has occurred
- PMID: 12084815
- PMCID: PMC123121
- DOI: 10.1073/pnas.142053899
Interactions between Eph kinases and ephrins provide a mechanism to support platelet aggregation once cell-to-cell contact has occurred
Abstract
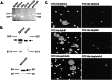
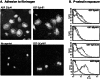
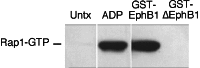
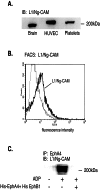
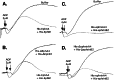
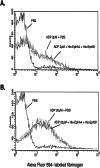
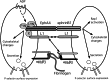
Eph kinases are receptor tyrosine kinases whose ligands, the ephrins, are also expressed on the surface of cells. Interactions between Eph kinases and ephrins on adjacent cells play a central role in neuronal patterning and vasculogenesis. Here we examine the expression of ephrins and Eph kinases on human blood platelets and explore their role in the formation of the hemostatic plug. The results show that human platelets express EphA4 and EphB1, and the ligand, ephrinB1. Forced clustering of EphA4 or ephrinB1 led to cytoskeletal reorganization, adhesion to fibrinogen, and alpha-granule secretion. Clustering of ephrinB1 also caused activation of the Ras family member, Rap1B. In platelets that had been activated by ADP and allowed to aggregate, EphA4 formed complexes with two tyrosine kinases, Fyn and Lyn, and the cell adhesion molecule, L1. Blockade of Eph/ephrin interactions prevented the formation of these complexes and caused platelet aggregation at low ADP concentrations to become more readily reversible. We propose that when sustained contacts between platelets have occurred in response to agonists such as collagen, ADP, and thrombin, the binding of ephrins to Eph kinases on adjacent platelets provides a mechanism to perpetuate signaling and promote stable platelet aggregation.
Figures

References
-
- Shattil S J. Thromb Haemostasis. 1999;82:318–325. - PubMed
-
- Angelillo-Scherrer A, De Frutos P G, Aparicio C, Melis E, Savi P, Lupu F, Arnout J, Dewerchin M, Hoylaerts M F, Herbert M, et al. Nat Med. 2001;7:215–221. - PubMed
-
- Wilkinson D G. Int Rev Cytol. 2000;196:177–244. - PubMed
-
- Dodelet V C, Pasquale E B. Oncogene. 2000;19:5614–5619. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

