Direct detection of potential selenium delivery proteins by using an Escherichia coli strain unable to incorporate selenium from selenite into proteins
- PMID: 12084818
- PMCID: PMC123109
- DOI: 10.1073/pnas.142291199
Direct detection of potential selenium delivery proteins by using an Escherichia coli strain unable to incorporate selenium from selenite into proteins
Abstract
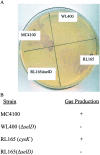
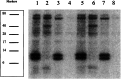
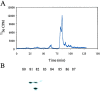
Selenium can be metabolized for protein synthesis by two major pathways in vivo. In a specific pathway it can be inserted into polypeptide chains as the amino acid selenocysteine, as directed by the UGA codon. Alternatively, selenium can be substituted for sulfur to generate the free amino acids selenocysteine and selenomethionine, and these are incorporated nonspecifically into proteins in place of cysteine and methionine, respectively. A mutant strain of Escherichia coli was constructed that is deficient in utilization of inorganic selenium for both specific and nonspecific pathways of selenoprotein synthesis. Disruption of the cysK gene prevented synthesis of free cysteine and selenocysteine from inorganic S and Se precursors. Inactivation of the selD gene prevented synthesis of selenophosphate, the reactive selenium donor, required for the specific incorporation pathway. As expected, the double mutant strain, RL165 Delta selD, when grown anaerobically in LB + glucose medium containing (75)SeO(3)(2-), failed to synthesize selenium-dependent formate dehydrogenase H and seleno-tRNAs. However, it incorporated 24% as much selenium as the wild-type strain. Selenium in the deficient strain was bound to five different proteins. A 39-kDa species was identified as glyceraldehyde-3-phosphate dehydrogenase. It is possible that selenium was bound as a perselenide derivative to the reactive cysteine residue of this enzyme. A 28-kDa protein identified as deoxyribose phosphate aldolase also contained bound selenium. These (75)Se-labeled proteins may have alternate roles as selenium delivery proteins.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

