IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth
- PMID: 12084823
- PMCID: PMC150776
- DOI: 10.1105/tpc.001388
IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth
Erratum in
-
CORRECTION: IRT1, an arabidopsis transporter essential for iron uptake from the soil and for plant growth.Plant Cell. 2021 Apr 17;33(2):439-440. doi: 10.1093/plcell/koaa033. Plant Cell. 2021. PMID: 33866371 Free PMC article. No abstract available.
Abstract
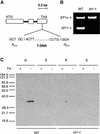
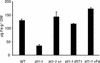
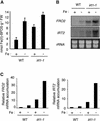
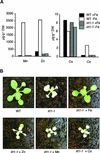
Plants are the principal source of iron in most diets, yet iron availability often limits plant growth. In response to iron deficiency, Arabidopsis roots induce the expression of the divalent cation transporter IRT1. Here, we present genetic evidence that IRT1 is essential for the uptake of iron from the soil. An Arabidopsis knockout mutant in IRT1 is chlorotic and has a severe growth defect in soil, leading to death. This defect is rescued by the exogenous application of iron. The mutant plants do not take up iron and fail to accumulate other divalent cations in low-iron conditions. IRT1-green fluorescent protein fusion, transiently expressed in culture cells, localized to the plasma membrane. We also show, through promoter::beta-glucuronidase analysis and in situ hybridization, that IRT1 is expressed in the external cell layers of the root, specifically in response to iron starvation. These results clearly demonstrate that IRT1 is the major transporter responsible for high-affinity metal uptake under iron deficiency.
Figures






Similar articles
-
Arabidopsis IRT2 cooperates with the high-affinity iron uptake system to maintain iron homeostasis in root epidermal cells.Planta. 2009 May;229(6):1171-9. doi: 10.1007/s00425-009-0904-8. Epub 2009 Feb 28. Planta. 2009. PMID: 19252923
-
Polarization of IRON-REGULATED TRANSPORTER 1 (IRT1) to the plant-soil interface plays crucial role in metal homeostasis.Proc Natl Acad Sci U S A. 2014 Jun 3;111(22):8293-8. doi: 10.1073/pnas.1402262111. Epub 2014 May 19. Proc Natl Acad Sci U S A. 2014. PMID: 24843126 Free PMC article.
-
The essential basic helix-loop-helix protein FIT1 is required for the iron deficiency response.Plant Cell. 2004 Dec;16(12):3400-12. doi: 10.1105/tpc.104.024315. Epub 2004 Nov 11. Plant Cell. 2004. PMID: 15539473 Free PMC article.
-
Birth, life and death of the Arabidopsis IRT1 iron transporter: the role of close friends and foes.Planta. 2022 Nov 11;256(6):112. doi: 10.1007/s00425-022-04018-7. Planta. 2022. PMID: 36367624 Review.
-
The zinc homeostasis network of land plants.Biochim Biophys Acta. 2012 Sep;1823(9):1553-67. doi: 10.1016/j.bbamcr.2012.05.016. Epub 2012 May 22. Biochim Biophys Acta. 2012. PMID: 22626733 Review.
Cited by
-
Searching iron sensors in plants by exploring the link among 2'-OG-dependent dioxygenases, the iron deficiency response and metabolic adjustments occurring under iron deficiency.Front Plant Sci. 2013 May 31;4:169. doi: 10.3389/fpls.2013.00169. eCollection 2013. Front Plant Sci. 2013. PMID: 23755060 Free PMC article.
-
ZAT10 plays dual roles in cadmium uptake and detoxification in Arabidopsis.Front Plant Sci. 2022 Aug 30;13:994100. doi: 10.3389/fpls.2022.994100. eCollection 2022. Front Plant Sci. 2022. PMID: 36110357 Free PMC article.
-
SlbHLH068 interacts with FER to regulate the iron-deficiency response in tomato.Ann Bot. 2015 Jul;116(1):23-34. doi: 10.1093/aob/mcv058. Epub 2015 Jun 12. Ann Bot. 2015. PMID: 26070639 Free PMC article.
-
Iron homeostasis in Arabidopsis thaliana: transcriptomic analyses reveal novel FIT-regulated genes, iron deficiency marker genes and functional gene networks.BMC Plant Biol. 2016 Oct 3;16(1):211. doi: 10.1186/s12870-016-0899-9. BMC Plant Biol. 2016. PMID: 27716045 Free PMC article.
-
The Intensity of Manganese Deficiency Strongly Affects Root Endodermal Suberization and Ion Homeostasis.Plant Physiol. 2019 Oct;181(2):729-742. doi: 10.1104/pp.19.00507. Epub 2019 Aug 9. Plant Physiol. 2019. PMID: 31399491 Free PMC article.
References
-
- Axelos, M., Curie, C., Mazzolini, L., Bardet, C., and Lescure, B. (1992). A protocol for transient expression in Arabidopsis thaliana protoplasts isolated from cell suspension culture. Plant Physiol. Biochem. 30, 123–128.
-
- Bechtold, N., Ellis, J., and Pelletier, G. (1993). In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C. R. Acad. Sci. Paris 316, 1194–1199.
-
- Bloomer, J.R., Reuter, R.J., Morton, K.O., and Wehner, J.M. (1983). Enzymatic formation of zinc-protoporphyrin by rat liver and its potential effect on hepatic heme metabolism. Gastroenterology 85, 663–668. - PubMed
-
- Bouchez, D., Camilleri, C., and Caboche, M. (1993). A binary vector based on Basta resistance for in planta transformation of Arabidopsis thaliana. C. R. Acad. Sci. Paris 316, 1188–1193.
-
- Bradford, M.M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

