Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation
- PMID: 12084831
- PMCID: PMC150784
- DOI: 10.1105/tpc.001263
Expression of the IRT1 metal transporter is controlled by metals at the levels of transcript and protein accumulation
Abstract
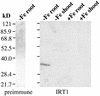
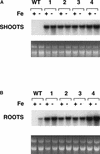
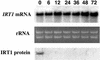
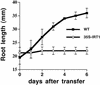
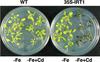
Iron, an essential nutrient, is not readily available to plants because of its low solubility. In addition, iron is toxic in excess, catalyzing the formation of hydroxyl radicals that can damage cellular constituents. Consequently, plants must carefully regulate iron uptake so that iron homeostasis is maintained. The Arabidopsis IRT1 gene is the major transporter responsible for high-affinity iron uptake from the soil. Here, we show that the steady state level of IRT1 mRNA was induced within 24 h after transfer of plants to iron-deficient conditions, with protein levels peaking 72 h after transfer. IRT1 mRNA and protein were undetectable 12 h after plants were shifted back to iron-sufficient conditions. Overexpression of IRT1 did not confer dominant gain-of-function enhancement of metal uptake. Analysis of 35S-IRT1 transgenic plants revealed that although IRT1 mRNA was expressed constitutively in these plants, IRT1 protein was present only in the roots when iron is limiting. Under these conditions, plants that overexpressed IRT1 accumulated higher levels of cadmium and zinc than wild-type plants, indicating that IRT1 is responsible for the uptake of these metals and that IRT1 protein levels are indeed increased in these plants. Our results suggest that the expression of IRT1 is controlled by two distinct mechanisms that provide an effective means of regulating metal transport in response to changing environmental conditions.
Figures
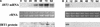


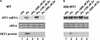


References
-
- Assuncao, A.G.L., Martins, P., De Folter, S., Vooijs, R., Schat, H., and Aarts, M.G.G. (2001). Elevated expression of metal transporter genes in three accessions of the metal hyperaccumulator Thlaspi caerulescens. Plant Cell Environ. 24, 217–226.
-
- Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., and Struhl, K. (2002). Current Protocols in Molecular Biology. (New York: John Wiley & Sons).
-
- Bent, A.F., Kunkel, B.N., Dahlbeck, D., Brown, K.L., Schmidt, R., Giraudat, J., Leung, J., and Staskawicz, B.J. (1994). RPS2 of Arabidopsis thaliana: A leucine-rich repeat class of plant disease resistance genes. Science 265, 1856–1860. - PubMed
-
- Briat, J.-F., and Lobréaux, S. (1997). Iron transport and storage in plants. Trends Plant Sci. 2, 187–193.
-
- Eckhardt, U., Marques, A.M., and Buckout, T.J. (2001). Two iron-regulated cation transporters from tomato complement metal uptake-deficient yeast mutants. Plant Mol. Biol. 45, 437–448. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

