Using deeply trapped intermediates to map the cytochrome c folding landscape
- PMID: 12084923
- PMCID: PMC124336
- DOI: 10.1073/pnas.132254499
Using deeply trapped intermediates to map the cytochrome c folding landscape
Abstract
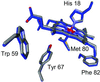
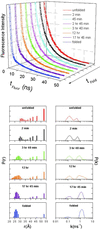
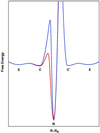
Replacement of iron with cobalt(III) selectively introduces a deep trap in the folding-energy landscape of the heme protein cytochrome c. Remarkably, neither the protein structure nor the folding thermodynamics is perturbed by this metal-ion substitution, as shown by data from spectroscopic and x-ray diffraction experiments. Through kinetics measurements, we have found parallel folding pathways involving several different misligated Co(III) species, and, as these folding intermediates persist for several hours under certain conditions, we have been able to elucidate fully their spectroscopic properties. The results, along with an analysis of the fluorescence energy-transfer kinetics during refolding, show that rapidly equilibrating populations of compact and extended polypeptide conformations are present until all molecules have reached the native structure. These measurements provide direct evidence that collapsed denatured structures are not substantially more stable than extended conformations of cytochrome c.
Figures


Similar articles
-
Folding of horse cytochrome c in the reduced state.J Mol Biol. 2001 Oct 5;312(5):1135-60. doi: 10.1006/jmbi.2001.4993. J Mol Biol. 2001. PMID: 11580255
-
Mapping the cytochrome C folding landscape.J Am Chem Soc. 2002 May 15;124(19):5481-5. doi: 10.1021/ja017399r. J Am Chem Soc. 2002. PMID: 11996590
-
Kinetics and motional dynamics of spin-labeled yeast iso-1-cytochrome c: 1. Stopped-flow electron paramagnetic resonance as a probe for protein folding/unfolding of the C-terminal helix spin-labeled at cysteine 102.Biochemistry. 1997 Mar 11;36(10):2884-97. doi: 10.1021/bi962155i. Biochemistry. 1997. PMID: 9062118
-
The folding energy landscape and free energy excitations of cytochrome c.Acc Chem Res. 2010 May 18;43(5):652-60. doi: 10.1021/ar9002703. Acc Chem Res. 2010. PMID: 20143816 Free PMC article. Review.
-
Early events, kinetic intermediates and the mechanism of protein folding in cytochrome C.Int J Mol Sci. 2009 Apr 1;10(4):1476-1499. doi: 10.3390/ijms10041476. Int J Mol Sci. 2009. PMID: 19468320 Free PMC article. Review.
Cited by
-
Biological inorganic chemistry at the beginning of the 21st century.Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):3563-8. doi: 10.1073/pnas.0730378100. Epub 2003 Mar 25. Proc Natl Acad Sci U S A. 2003. PMID: 12657732 Free PMC article. Review.
-
Structural analysis of zinc-substituted cytochrome c.J Biol Inorg Chem. 2003 Apr;8(4):394-400. doi: 10.1007/s00775-002-0428-1. Epub 2002 Dec 14. J Biol Inorg Chem. 2003. PMID: 12761660
-
Hg(II) binding to a weakly associated coiled coil nucleates an encoded metalloprotein fold: a kinetic analysis.Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):3760-5. doi: 10.1073/pnas.0336055100. Epub 2003 Jan 27. Proc Natl Acad Sci U S A. 2003. PMID: 12552128 Free PMC article.
-
Characterization of Intramolecular Interactions of Cytochrome c Using Hydrogen-Deuterium Exchange-Trapped Ion Mobility Spectrometry-Mass Spectrometry and Molecular Dynamics.Anal Chem. 2017 Sep 5;89(17):8757-8765. doi: 10.1021/acs.analchem.7b00844. Epub 2017 Aug 11. Anal Chem. 2017. PMID: 28742962 Free PMC article.
-
Toward resolution of ambiguity for the unfolded state.Biophys J. 2008 Jul;95(2):503-9. doi: 10.1529/biophysj.107.121855. Epub 2008 May 9. Biophys J. 2008. PMID: 18469075 Free PMC article.
References
-
- Onuchic J N, Nymeyer H, Garcia A E, Chahine J, Socci N D. Adv Protein Chem. 2000;53:87–152. - PubMed
-
- Portman J J, Takada S, Wolynes P G. J Chem Phys. 2001;114:5069–5081.
-
- Portman J J, Takada S, Wolynes P G. J Chem Phys. 2001;114:5082–5096.
-
- Özkan S B, Bahar I, Dill K A. Nat Struct Biol. 2001;8:765–769. - PubMed
-
- Mirny L, Shakhnovich E I. Annu Rev Biophys Biomol Struct. 2001;30:361–396. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

