Tissue levels of active matrix metalloproteinase-2 and -9 in colorectal cancer
- PMID: 12085179
- PMCID: PMC2375422
- DOI: 10.1038/sj.bjc.6600366
Tissue levels of active matrix metalloproteinase-2 and -9 in colorectal cancer
Abstract
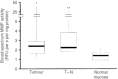
The bioactivity of matrix metalloproteinases was studied in tissues from colorectal cancer patients by means of both quantitative gelatin zymography and a fluorometric activity assay. Next to paired samples of tumour tissue and distant normal mucosa (n=73), transitional tissue was analysed from a limited (n=33) number of patients. Broad-spectrum matrix metalloproteinase activity and both the active and latent forms of the gelatinases matrix metalloproteinase-2 and -9 were higher in tumour than in normal mucosa. The ratio's between active and latent forms of matrix metalloproteinase-2 and -9 were highest in tumour tissue and normal mucosa, respectively. Matrix metalloproteinase-2 levels, both active and latent forms, correlated inversely with stage of disease, the tumours without synchronous distant metastases containing significantly (P=0.005) more active matrix metalloproteinase-2 than the others. At much lower levels of activity, the same trend was observed in distant normal mucosa. The level of latent form of matrix metalloproteinase-9 in tumour depended on tumour location. Neither the active form of matrix metalloproteinase-9 nor broad-spectrum matrix metalloproteinase activity in tumour tissue did correlate with any of the clinicopathological parameters investigated. The results demonstrate explicit differences between the activity of matrix metalloproteinase-2 and -9, indicating different roles for both gelatinases in tumour progression. Such data are necessary in order to develop rational anti-cancer therapies based on inhibition of specific matrix metalloproteinases.
Copyright 2002 Cancer Research UK
Figures
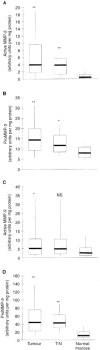
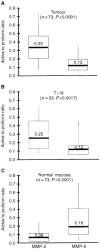
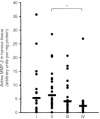
References
-
- BakerEABerginFGLeaperDJ2000Matrix metalloproteinases, their tissue inhibitors and colorectal cancer staging Br J Surg 8712151221 - PubMed
-
- BeekmanBDrijfhoutJWRondayHKTeKoppeleJM1999Fluorogenic MMP activity assay for plasma including MMPs complexed to alpha 2-macroglobulin Ann NY Acad Sci 878150158 - PubMed
-
- CurranSMurrayGI2000Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis Eur J Cancer 3616211630 - PubMed
-
- DeClerckYA2000Interactions between tumour cells and stromal cells and proteolytic modification of the extracellular matrix by metalloproteinases in cancer Eur J Cancer 3612581268 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

