Alternating dose-dense chemotherapy in patients with high volume disseminated non-seminomatous germ cell tumours
- PMID: 12085204
- PMCID: PMC2746595
- DOI: 10.1038/sj.bjc.6600272
Alternating dose-dense chemotherapy in patients with high volume disseminated non-seminomatous germ cell tumours
Abstract
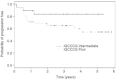
Only about half of patients with a poor-prognosis non-seminomatous germ-cell tumours can achieve a cure. The aim of this phase II study was to assess the efficacy and toxicity of a dose-dense alternating chemotherapy regimen in this subset of patients. High volume non-seminomatous germ-cell tumours was defined as follows: at least two sites of non pulmonary metastases, an extragonadal primary tumour, a serum human chorionic gonadotropin level higher than 10 000 mIU x ml(-1), or a alpha-foetoprotein level higher than 2000 mIU ml(-1). Patients who fulfilled these criteria were treated with the so-called BOP-CISCA-POMB-ACE regimen (bleomycin, vincristine, and cisplatin; cisplatin, cyclophosphamide, and doxorubicin; cisplatin, vincristine, methotrexate, and bleomycin; etoposide, dactinomycin, and cyclophosphamide) plus granulocyte colony-stimulating factor. A total of 58 patients were enrolled. Patients were retrospectively classified according to the International Germ-Cell Cancer Consensus Group classification; 38 patients (66%) had poor-prognosis disease and 19 patients (33%) had intermediate-prognosis. Patients received a median of 2.5 courses (range 0.25 to five courses) of the BOP-CISCA-POMB-ACE regimen. Forty-two patients (72.4%) had a complete response to therapy. With a median follow-up time of 31 months, the 3-year progression-free survival rate was 71% (95% confidence interval, 60 to 84%) and the 3-year overall survival rate was 73% (95% confidence interval: 62 to 86%). The 3-year PFS rates were 83% (95% confidence interval: 68 to 100%) in the intermediate-prognosis group and 65% (95% confidence interval: 51 to 82%) in the poor-prognosis group. Early side effects included mainly grade 4 haematologic toxicity (neutropaenia in 79% of patients, thrombocytopaenia in 69%, anaemia in 22%), grade 4 stomatitis (19%), and four early deaths (7% of patients), at least partially related to toxicity. The dose-dense BOP-CISCA-POMB-ACE regimen is highly active in patients with non-seminomatous germ-cell tumours classified as intermediate-prognosis or poor-prognosis according to the International Germ-Cell Cancer Consensus Group. Because outcomes with this regimen compare favourably with outcome after standard therapy, dose-dense chemotherapy should be further investigated in this subset of patients.
comCopyright 2002 Cancer Research UK
Figures
Similar articles
-
The management and survival of patients with advanced germ-cell tumours: improving outcome in intermediate and poor prognosis patients.Clin Oncol (R Coll Radiol). 2004 Feb;16(1):40-7. doi: 10.1016/s0936-6555(03)00166-3. Clin Oncol (R Coll Radiol). 2004. PMID: 14768754
-
Treatment of men with metastatic non-seminomatous germ cell tumours with cyclical POMB/ACE chemotherapy.Ann Oncol. 1997 May;8(5):477-83. doi: 10.1023/a:1008279222625. Ann Oncol. 1997. PMID: 9233528
-
Long-term results of first-line sequential high-dose etoposide, ifosfamide, and cisplatin chemotherapy plus autologous stem cell support for patients with advanced metastatic germ cell cancer: an extended phase I/II study of the German Testicular Cancer Study Group.J Clin Oncol. 2003 Nov 15;21(22):4083-91. doi: 10.1200/JCO.2003.09.035. Epub 2003 Oct 20. J Clin Oncol. 2003. PMID: 14568987 Clinical Trial.
-
Intensive induction chemotherapy with CBOP/BEP in patients with poor prognosis germ cell tumors.J Clin Oncol. 2003 Mar 1;21(5):871-7. doi: 10.1200/JCO.2003.05.155. J Clin Oncol. 2003. PMID: 12610187 Review.
-
The use of dose-intensified chemotherapy in the treatment of metastatic nonseminomatous testicular germ cell tumors. German Testicular Cancer Study Group.Semin Oncol. 1998 Apr;25(2 Suppl 4):24-32; discussion 45-8. Semin Oncol. 1998. PMID: 9578059 Review.
Cited by
-
Advances in the treatment of testicular cancer.Drugs. 2006;66(5):641-59. doi: 10.2165/00003495-200666050-00005. Drugs. 2006. PMID: 16620142 Review.
-
Personalised chemotherapy based on tumour marker decline in poor prognosis germ-cell tumours (GETUG 13): a phase 3, multicentre, randomised trial.Lancet Oncol. 2014 Dec;15(13):1442-1450. doi: 10.1016/S1470-2045(14)70490-5. Epub 2014 Nov 13. Lancet Oncol. 2014. PMID: 25456363 Free PMC article. Clinical Trial.
-
Intensive induction chemotherapy with C-BOP/BEP for intermediate- and poor-risk metastatic germ cell tumours (EORTC trial 30948).Br J Cancer. 2005 Nov 28;93(11):1209-14. doi: 10.1038/sj.bjc.6602830. Br J Cancer. 2005. PMID: 16251877 Free PMC article. Clinical Trial.
References
-
- AndréFFizaziKCulineSDrozJPTaupinPLhommeCTerrier-LacombeMThéodoreC2000The growing teratoma syndrome: results of therapy and long-term follow-up of a rare complication of non seminomatous germ-cell tumors Eur J Cancer 3613891394 - PubMed
-
- BaumeDPicoJLDrozJPOstronoffMAzabMGillesEGhosnMSalloumEGouyetteABeaujeanF1990Value of high-dose chemotherapy followed by bone marrow autograft in non-seminomatous germinal tumor with poor prognosis. Results of the combination of cisplatinum, etoposide and cyclophosphamide Bull Cancer 77169180 - PubMed
-
- BokemeyerCSchmollHJ1995Treatment of testicular cancer and the development of secondary malignancies J Clin Oncol 13283292 - PubMed
-
- BokemeyerCKollmannsbergerCMeisnerCHarstrickABeyerJMetznerBHartmannJTSchmollHJEinhornLKanzLNicholsC1999First-line high-dose chemotherapy compared with standard-dose PEB/VIP chemotherapy in patients with advanced germ cell tumors: A multivariate and matched-pair analysis J Clin Oncol 1734503456 - PubMed
-
- BoslGJMotzerRJ1997Testicular germ-cell cancer N Engl J Med 337242253 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous