Apical barriers to airway epithelial cell gene transfer with amphotropic retroviral vectors
- PMID: 12085240
- PMCID: PMC7091907
- DOI: 10.1038/sj.gt.3301714
Apical barriers to airway epithelial cell gene transfer with amphotropic retroviral vectors
Abstract
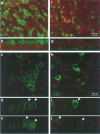
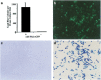
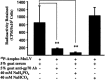
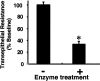
Gene transfer to airway epithelia with amphotropic pseudotyped retroviral vectors is inefficient following apical vector application. To better understand this inefficiency, we localized the expression of Pit2, the amphotropic receptor, in polarized human airway epithelia. Pit2 was expressed on both the apical and basolateral surfaces of the cells, suggesting that factors other than receptor abundance may limit apical gene transfer efficiency. Binding studies performed with radiolabeled amphotropic MuLV suggested that the apically applied virus binds to Pit2. Hypothetical barriers to retroviral gene transfer include the apical glycocalyx and other secreted products of epithelia. In this study, we demonstrated that sialic acid, keratan sulfate and collagen type V are present on the apical surface of well-differentiated human airway epithelia. While enzyme treatment reduced the abundance of these components, the treatment also decreased the transepithelial resistance to approximately 35% of the controls, suggesting that the epithelial integrity was impaired. To attain an airway epithelial culture with a modified apical surface and intact epithelial integrity, we utilized 100 mM 2-deoxy-D-glucose, a glycosylation inhibitor, to prevent the glycocalyx from reforming following enzyme treatment. This approach allowed the resistance, but not the apical glycocalyx to recover. Despite this physical modification of the cell surface, the amphotropic retroviral vector failed to transduce airway epithelia following apical application. These results suggest that factors other than apical receptor abundance and the glycocalyx inhibit amphotropic retroviral gene transfer in human airway epithelia.
Figures




References
-
- Wang G, Sinn PL, McCray PB., Jr Development of retroviral vectors for gene transfer to airway epithelia. Curr Opin Mol Ther. 2000;2:497–506. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

