Pre- and postsynaptic volatile anaesthetic actions on glycinergic transmission to spinal cord motor neurons
- PMID: 12086976
- PMCID: PMC1573392
- DOI: 10.1038/sj.bjp.0704760
Pre- and postsynaptic volatile anaesthetic actions on glycinergic transmission to spinal cord motor neurons
Abstract
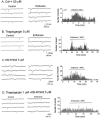
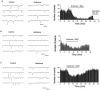
1. A common anaesthetic endpoint, prevention of withdrawal from a noxious stimulus, is determined primarily in spinal cord, where glycine is an important inhibitory transmitter. To define pre- and postsynaptic anaesthetic actions at glycinergic synapses, the effects of volatile anaesthetic agents on spontaneous and evoked glycinergic currents in spinal cord motor neurons from 6 - 14-day old rats was investigated. 2. The volatile anaesthetic agents enflurane, isoflurane and halothane significantly increased the frequency of glycinergic mIPSCs, enflurane to 190.4% of control+/-22.0 (mean+/-s.e.m., n=7, P<0.01), isoflurane to 199.0%+/-28.8 (n=7, P<0.05) and halothane to 198.2%+/-19.5 (n=7, P<0.01). However without TTX, isoflurane and halothane had no significant effect and enflurane decreased sIPSC frequency to 42.5% of control+/-12.4 (n=6, P<0.01). All the anaesthetics prolonged the decay time constant (tau) of both spontaneous and glycine-evoked currents without increasing amplitude. With TTX total charge transfer was increased; without TTX charge transfer was unchanged (isoflurane and halothane) or decreased (enflurane). 3. Enflurane-induced mIPSC frequency increases were not significantly affected by Cd(2+) (50 microM), thapsigargin (1 - 5 microM), or KB-R7943 (5 microM). KB-R7943 and thapsigargin together abolished the enflurane-induced increase in mIPSC frequency. 4. There are opposing facilitatory and inhibitory actions of volatile anaesthetics on glycine release dependent on calcium homeostatic mechanisms and sodium channels respectively. Under normal conditions (no TTX) the absolute amount of glycinergic inhibition does not increase. The contribution of glycinergic inhibition to anaesthesia may depend on its duration rather than its absolute magnitude.
Figures






Similar articles
-
Enflurane decreases glutamate neurotransmission to spinal cord motor neurons by both pre- and postsynaptic actions.Anesth Analg. 2003 May;96(5):1354-1359. doi: 10.1213/01.ANE.0000055649.06649.D2. Anesth Analg. 2003. PMID: 12707133
-
Enflurane directly depresses glutamate AMPA and NMDA currents in mouse spinal cord motor neurons independent of actions on GABAA or glycine receptors.Anesthesiology. 2000 Oct;93(4):1075-84. doi: 10.1097/00000542-200010000-00032. Anesthesiology. 2000. PMID: 11020764
-
Transition from GABAergic to glycinergic synaptic transmission in newly formed spinal networks.J Neurophysiol. 2001 Jul;86(1):492-502. doi: 10.1152/jn.2001.86.1.492. J Neurophysiol. 2001. PMID: 11431527
-
The effects of volatile anesthetics on synaptic and extrasynaptic GABA-induced neurotransmission.Brain Res Bull. 2013 Apr;93:69-79. doi: 10.1016/j.brainresbull.2012.08.001. Epub 2012 Aug 17. Brain Res Bull. 2013. PMID: 22925739 Review.
-
[Inhibitory action of sensory transmission by inhalational anesthetics in the spinal cord].Masui. 2003 Mar;52(3):240-50. Masui. 2003. PMID: 12703065 Review. Japanese.
Cited by
-
Mitochondrial Function and Anesthetic Sensitivity in the Mouse Spinal Cord.Anesthesiology. 2021 Jun 1;134(6):901-914. doi: 10.1097/ALN.0000000000003794. Anesthesiology. 2021. PMID: 33909880 Free PMC article.
-
Structure and Pharmacologic Modulation of Inhibitory Glycine Receptors.Mol Pharmacol. 2016 Sep;90(3):318-25. doi: 10.1124/mol.116.105726. Epub 2016 Jul 11. Mol Pharmacol. 2016. PMID: 27401877 Free PMC article. Review.
-
Allosteric modulation of glycine receptors.Br J Pharmacol. 2011 Sep;164(2):224-36. doi: 10.1111/j.1476-5381.2011.01471.x. Br J Pharmacol. 2011. PMID: 21557733 Free PMC article. Review.
-
Ethanol withdrawal hyper-responsiveness mediated by NMDA receptors in spinal cord motor neurons.Br J Pharmacol. 2003 May;139(1):73-80. doi: 10.1038/sj.bjp.0705198. Br J Pharmacol. 2003. PMID: 12746225 Free PMC article.
-
Ethanol effects on glycinergic transmission: From molecular pharmacology to behavior responses.Pharmacol Res. 2015 Nov;101:18-29. doi: 10.1016/j.phrs.2015.07.002. Epub 2015 Jul 6. Pharmacol Res. 2015. PMID: 26158502 Free PMC article. Review.
References
-
- ANTKOWIAK B., HECK D. Effects of the volatile anesthetic enflurane on spontaneous discharge rate and GABA(A)-mediated inhibition of Purkinje cells in rat cerebellar slices. J. Neurophysiol. 1997;77:2525–2538. - PubMed
-
- ANTOGNINI J.F. The relationship among brain, spinal cord and anesthetic requirements. Med. Hypotheses. 1997;48:83–87. - PubMed
-
- ANTOGNINI J.F., SCHWARTZ K. Exaggerated anesthetic requirements in the preferentially anesthetized brain. Anesthesiology. 1993;79:1244–1249. - PubMed
-
- BAI D., ZHU G., PENNEFATHER P., JACKSON M.F., MACDONALD J.F., ORSER B.A. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(A) receptors in hippocampal neurons. Mol. Pharmacol. 2001;59:814–824. - PubMed
-
- BANKS M.I., PEARCE R.A. Dual actions of volatile anesthetics on GABA(A) IPSCs: dissociation of blocking and prolonging effects. Anesthesiology. 1999;90:124–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

