Tonic inhibitory control exerted by opioid peptides in the paraventricular nuclei of the hypothalamus on regional hemodynamic activity in rats
- PMID: 12086985
- PMCID: PMC1573405
- DOI: 10.1038/sj.bjp.0704780
Tonic inhibitory control exerted by opioid peptides in the paraventricular nuclei of the hypothalamus on regional hemodynamic activity in rats
Abstract
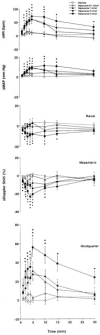
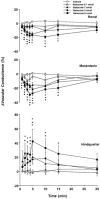
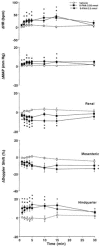
1. Systemic and regional cardiovascular changes were measured following bilateral microinjection of specific and selective opioid-receptor antagonists into the paraventricular nuclei of the hypothalamus (PVN) of awake, freely moving rats. 2. PVN microinjection of increasing doses of the specific opioid antagonist naloxone - methiodide (1 - 5.0 nmol), or a selective mu-opioid receptor antagonist, beta-funaltrexamine (0.05 - 0.5 nmol), evoked important cardiovascular changes characterized by small and transient increases in heart rate (HR) and mean arterial pressure (MAP), vasoconstriction in renal and superior mesenteric vascular beds and vasodilation in the hindquarter vascular bed. 3. No significant cardiovascular changes were observed following PVN administration of the highly selective delta-opioid-receptor antagonist, ICI 174864 (0.1 - 1 nmol), or the selective kappa-opioid-receptor antagonist, nor-binaltorphine (0.1 - 1 nmol). 4. Most of the cardiovascular responses to naloxone (3 nmol) and beta-funaltrexamine (0.5 nmol) were attenuated or abolished by an i.v. treatment with a specific vasopressin V(1) receptor antagonist. 5. These results suggest that endogenous opioid peptides and mu-type PVN opioid receptors modulate a tonically-active central depressor pathway acting on systemic and regional haemodynamic systems. Part of this influence could involve a tonic inhibition of vasopressin release.
Figures



Similar articles
-
Mechanisms of the regional hemodynamic effects of a mu-opioid receptor agonist microinjected into the hypothalamic paraventricular nuclei of conscious unrestrained rats.J Pharmacol Exp Ther. 1997 Jan;280(1):460-70. J Pharmacol Exp Ther. 1997. PMID: 8996229
-
Regional haemodynamic effects of mu-, delta-, and kappa-opioid agonists microinjected into the hypothalamic paraventricular nuclei of conscious, unrestrained rats.Br J Pharmacol. 1995 Jun;115(4):613-21. doi: 10.1111/j.1476-5381.1995.tb14976.x. Br J Pharmacol. 1995. PMID: 7582480 Free PMC article.
-
Effects of mu-opioid receptor stimulation in the hypothalamic paraventricular nucleus on basal and stress-induced catecholamine secretion and cardiovascular responses.J Pharmacol Exp Ther. 1986 Dec;239(3):814-22. J Pharmacol Exp Ther. 1986. PMID: 3025420
-
Stress-specific opioid modulation of haemodynamic counter-regulation.Clin Exp Pharmacol Physiol. 2002 Mar;29(3):248-53. doi: 10.1046/j.1440-1681.2002.03638.x. Clin Exp Pharmacol Physiol. 2002. PMID: 11906493 Review.
-
Fostering orphan receptors: an indispensable role for integrative, in vivo, haemodynamic studies.Curr Opin Pharmacol. 2003 Apr;3(2):140-5. doi: 10.1016/s1471-4892(03)00004-3. Curr Opin Pharmacol. 2003. PMID: 12681235 Review.
Cited by
-
Research trends of acupuncture therapy for hypertension over the past two decades: a bibliometric analysis.Cardiovasc Diagn Ther. 2023 Feb 28;13(1):67-82. doi: 10.21037/cdt-22-480. Epub 2022 Dec 15. Cardiovasc Diagn Ther. 2023. PMID: 36864974 Free PMC article. Review.
-
The Mechanism of Acupuncture in Treating Essential Hypertension: A Narrative Review.Int J Hypertens. 2019 Mar 7;2019:8676490. doi: 10.1155/2019/8676490. eCollection 2019. Int J Hypertens. 2019. PMID: 30984420 Free PMC article. Review.
-
Moxibustion Modulates Sympathoexcitatory Cardiovascular Reflex Responses Through Paraventricular Nucleus.Front Neurosci. 2019 Jan 21;12:1057. doi: 10.3389/fnins.2018.01057. eCollection 2018. Front Neurosci. 2019. Retraction in: Front Neurosci. 2021 Feb 08;14:630045. doi: 10.3389/fnins.2020.630045. PMID: 30718997 Free PMC article. Retracted.
-
Paraventricular Nucleus Modulates Excitatory Cardiovascular Reflexes during Electroacupuncture.Sci Rep. 2016 May 16;6:25910. doi: 10.1038/srep25910. Sci Rep. 2016. PMID: 27181844 Free PMC article.
-
Transgenerational blunting of morphine-induced corticosterone secretion is associated with dysregulated gene expression in male offspring.Brain Res. 2018 Jan 15;1679:19-25. doi: 10.1016/j.brainres.2017.11.004. Epub 2017 Nov 10. Brain Res. 2018. PMID: 29129606 Free PMC article.
References
-
- APPEL N.M., KIRITSY-ROY J.A., VAN LOON G.R. μ-opioid receptors at discrete hypothalamic and brainstem sites mediate opioid peptide induced increases in central sympathetic out flow. Brain Res. 1986;378:8–20. - PubMed
-
- ARNAULD E., CIRINO M., LAYTON B.S., RENAULD L.P. Contrasting actions of amino acids, acetylcholine, noradrenaline and leucine enkephalin on the excitability of supraoptic vasopressin-secreting neurons. Neuroendocrinol. 1983;36:187–196. - PubMed
-
- BACHELARD H., PITRE M., LESSARD A. Mechanisms of the regional hemodynamic effects of a mu-opioid receptor agonist microinjected into the hypothalamic paraventricular nuclei of conscious unrestrained rats. J. Pharmacol. Exp. Ther. 1997;280:460–470. - PubMed
-
- BOULOUX P.M.G.Cardiovascular responses to stress: the role of opioid peptides Baillière's Clinical endocrinology and metabolism 1987London: Baillière Tindall; 439–465.ed. Grossman, A., pp - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

