NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle
- PMID: 12086987
- PMCID: PMC1573403
- DOI: 10.1038/sj.bjp.0704778
NO- and haem-independent activation of soluble guanylyl cyclase: molecular basis and cardiovascular implications of a new pharmacological principle
Abstract
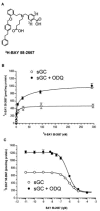
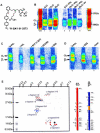
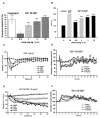
1. Soluble guanylyl cyclase (sGC) is the only proven receptor for the ubiquitous biological messenger nitric oxide (NO) and is intimately involved in many signal transduction pathways, most notably in regulating vascular tone and platelet function. sGC is a heterodimeric (alpha/ss) protein that converts GTP to cyclic GMP; NO binds to its prosthetic haem group. Here, we report the discovery of a novel sGC activating compound, its interaction with a previously unrecognized regulatory site and its therapeutic implications. 2. Through a high-throughput screen we identified BAY 58-2667, an amino dicarboxylic acid which potently activates sGC in an NO-independent manner. In contrast to NO, YC-1 and BAY 41-2272, the sGC stimulators described recently, BAY 58-2667 activates the enzyme even after it has been oxidized by the sGC inhibitor ODQ or rendered haem deficient. 3. Binding studies with radiolabelled BAY 58-2667 show a high affinity site on the enzyme. 4. Using photoaffinity labelling studies we identified the amino acids 371 (alpha-subunit) and 231 - 310 (ss-subunit) as target regions for BAY 58-2667. 5. sGC activation by BAY 58-2667 results in an antiplatelet activity both in vitro and in vivo and a potent vasorelaxation which is not influenced by nitrate tolerance. 6. BAY 58-2667 shows a potent antihypertensive effect in conscious spontaneously hypertensive rats. In anaesthetized dogs the hemodynamic effects of BAY 58-2667 and GTN are very similar on the arterial and venous system. 7. This novel type of sGC activator is a valuable research tool and may offer a new approach for treating cardiovascular diseases.
Figures


Comment in
-
Soluble guanylate cyclase: an old therapeutic target re-visited.Br J Pharmacol. 2002 Jul;136(5):637-40. doi: 10.1038/sj.bjp.0704779. Br J Pharmacol. 2002. PMID: 12086972 Free PMC article. No abstract available.
Similar articles
-
Distinct molecular requirements for activation or stabilization of soluble guanylyl cyclase upon haem oxidation-induced degradation.Br J Pharmacol. 2009 Jul;157(5):781-95. doi: 10.1111/j.1476-5381.2009.00263.x. Epub 2009 May 18. Br J Pharmacol. 2009. PMID: 19466990 Free PMC article.
-
Nitric oxide-independent stimulation of soluble guanylate cyclase with BAY 41-2272 in cardiovascular disease.Cardiovasc Drug Rev. 2007 Spring;25(1):30-45. doi: 10.1111/j.1527-3466.2007.00003.x. Cardiovasc Drug Rev. 2007. PMID: 17445086 Review.
-
Activation of haem-oxidized soluble guanylyl cyclase with BAY 60-2770 in human platelets lead to overstimulation of the cyclic GMP signaling pathway.PLoS One. 2012;7(11):e47223. doi: 10.1371/journal.pone.0047223. Epub 2012 Nov 8. PLoS One. 2012. PMID: 23144808 Free PMC article.
-
NO-independent, haem-dependent soluble guanylate cyclase stimulators.Handb Exp Pharmacol. 2009;(191):277-308. doi: 10.1007/978-3-540-68964-5_13. Handb Exp Pharmacol. 2009. PMID: 19089334 Review.
-
The fibrate gemfibrozil is a NO- and haem-independent activator of soluble guanylyl cyclase: in vitro studies.Br J Pharmacol. 2015 May;172(9):2316-29. doi: 10.1111/bph.13055. Epub 2015 Feb 10. Br J Pharmacol. 2015. PMID: 25536881 Free PMC article.
Cited by
-
Role of sGC-dependent NO signalling and myocardial infarction risk.J Mol Med (Berl). 2015 Apr;93(4):383-94. doi: 10.1007/s00109-015-1265-3. Epub 2015 Mar 4. J Mol Med (Berl). 2015. PMID: 25733135 Review.
-
Inhaled mosliciguat (BAY 1237592): targeting pulmonary vasculature via activating apo-sGC.Respir Res. 2022 Oct 1;23(1):272. doi: 10.1186/s12931-022-02189-1. Respir Res. 2022. PMID: 36183104 Free PMC article.
-
Decoding signaling mechanisms: unraveling the targets of guanylate cyclase agonists in cardiovascular and digestive diseases.Front Pharmacol. 2023 Dec 20;14:1272073. doi: 10.3389/fphar.2023.1272073. eCollection 2023. Front Pharmacol. 2023. PMID: 38186653 Free PMC article. Review.
-
Translating the oxidative stress hypothesis into the clinic: NOX versus NOS.J Mol Med (Berl). 2009 Nov;87(11):1071-6. doi: 10.1007/s00109-009-0544-2. Epub 2009 Oct 16. J Mol Med (Berl). 2009. PMID: 19834654 Free PMC article. Review.
-
NO-ferroheme is a signaling entity in the vasculature.Nat Chem Biol. 2023 Oct;19(10):1267-1275. doi: 10.1038/s41589-023-01411-5. Epub 2023 Sep 14. Nat Chem Biol. 2023. PMID: 37710073 Free PMC article.
References
-
- AHR H.J., STEINKE W. Imaging plate: validation and first experience with quantitative studies in whole-body autoradiography during drug development. Xenobiotic Metab. Dispos. 1994;9:371–378.
-
- ALONSO-ALIJA C., HEIL M., FLUBACHER D., NAAB P., STASCH J.-P., WUNDER F., DEMBOWSKY K., PERZBORN E., STAHL E.Novel derivatives of dicarboxylic acid having pharmaceutical properties 2001. WO-119780-A 2001.03.22
-
- BECKER E.M., SCHMIDT P., SCHRAMM M., SCHRÖDER H., WALTER U., HOENICKA M., GERZER R., STASCH J.-P. The vasodilator-stimulated phosphoprotein VASP: mediator of YC-1 and nitric oxide effects in human and rat platelets. J. Cardiovasc. Pharmacol. 2000;35:390–397. - PubMed
-
- BECKER E.M., WUNDER F., KAST R., ROBYR C., HOENICKA M., GERZER R., SCHRÖDER H., STASCH J.-P. Generation and characterization of a stable soluble guanylate cyclase overexpressing CHO cell line. Nitric Oxide. 1999;3:55–66. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

