Bacterial surface association of heat-labile enterotoxin through lipopolysaccharide after secretion via the general secretory pathway
- PMID: 12087095
- PMCID: PMC4391702
- DOI: 10.1074/jbc.M203740200
Bacterial surface association of heat-labile enterotoxin through lipopolysaccharide after secretion via the general secretory pathway
Abstract
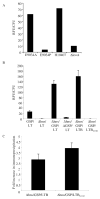
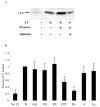
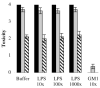
Heat-labile enterotoxin (LT) is an important virulence factor expressed by enterotoxigenic Escherichia coli. The route of LT secretion through the outer membrane and the cellular and extracellular localization of secreted LT were examined. Using a fluorescently labeled receptor, LT was found to be specifically secreted onto the surface of wild type enterotoxigenic Escherichia coli. The main terminal branch of the general secretory pathway (GSP) was necessary and sufficient to localize LT to the bacterial surface in a K-12 strain. LT is a heteromeric toxin, and we determined that its cell surface localization was mediated by the its B subunit independent of an intact G(M1) ganglioside binding site and that LT binds lipopolysaccharide and G(M1) concurrently. The majority of LT secreted into the culture supernatant by the GSP in E. coli associated with vesicles. Only a mutation in hns, not overexpression of the GSP or LT, caused an increase in vesicle yield, supporting a specific vesicle formation machinery regulated by the nucleoid-associated protein HNS. We propose a model in which LT is secreted by the GSP across the outer membrane, secreted LT binds lipopolysaccharide via a G(M1)-independent binding region on its B subunit, and LT on the surface of released outer membrane vesicles interacts with host cell receptors, leading to intoxication. These data explain a novel mechanism of vesicle-mediated receptor-dependent delivery of a bacterial toxin into a host cell.
Figures

Similar articles
-
Heat-labile enterotoxin: beyond G(m1) binding.Toxins (Basel). 2010 Jun;2(6):1445-70. doi: 10.3390/toxins2061445. Epub 2010 Jun 14. Toxins (Basel). 2010. PMID: 22069646 Free PMC article. Review.
-
Lipopolysaccharide 3-deoxy-D-manno-octulosonic acid (Kdo) core determines bacterial association of secreted toxins.J Biol Chem. 2004 Feb 27;279(9):8070-5. doi: 10.1074/jbc.M308633200. Epub 2003 Dec 4. J Biol Chem. 2004. PMID: 14660669 Free PMC article.
-
Context-dependent activation kinetics elicited by soluble versus outer membrane vesicle-associated heat-labile enterotoxin.Infect Immun. 2011 Sep;79(9):3760-9. doi: 10.1128/IAI.05336-11. Epub 2011 Jun 27. Infect Immun. 2011. PMID: 21708992 Free PMC article.
-
Residues of heat-labile enterotoxin involved in bacterial cell surface binding.J Bacteriol. 2009 May;191(9):2917-25. doi: 10.1128/JB.01622-08. Epub 2009 Mar 6. J Bacteriol. 2009. PMID: 19270095 Free PMC article.
-
Review of Newly Identified Functions Associated With the Heat-Labile Toxin of Enterotoxigenic Escherichia coli.Front Cell Infect Microbiol. 2019 Aug 13;9:292. doi: 10.3389/fcimb.2019.00292. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31456954 Free PMC article. Review.
Cited by
-
Membrane vesicles shed by Legionella pneumophila inhibit fusion of phagosomes with lysosomes.Infect Immun. 2006 Jun;74(6):3285-95. doi: 10.1128/IAI.01382-05. Infect Immun. 2006. PMID: 16714556 Free PMC article.
-
Differential Packaging Into Outer Membrane Vesicles Upon Oxidative Stress Reveals a General Mechanism for Cargo Selectivity.Front Microbiol. 2021 Jul 2;12:561863. doi: 10.3389/fmicb.2021.561863. eCollection 2021. Front Microbiol. 2021. PMID: 34276573 Free PMC article.
-
Production of outer membrane vesicles by the plague pathogen Yersinia pestis.PLoS One. 2014 Sep 8;9(9):e107002. doi: 10.1371/journal.pone.0107002. eCollection 2014. PLoS One. 2014. PMID: 25198697 Free PMC article.
-
No direct binding of the heat-labile enterotoxin of Escherichia coli to E. coli lipopolysaccharides.Glycoconj J. 2010 Jan;27(1):171-9. doi: 10.1007/s10719-009-9264-7. Epub 2009 Oct 21. Glycoconj J. 2010. PMID: 19844789
-
Comparative study of pathogenic and non-pathogenic Escherichia coli outer membrane vesicles and prediction of host-interactions with TLR signaling pathways.BMC Res Notes. 2018 Aug 1;11(1):539. doi: 10.1186/s13104-018-3648-3. BMC Res Notes. 2018. PMID: 30068381 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

