Role of the short isoform of myosin light chain kinase in the contraction of cultured smooth muscle cells as examined by its down-regulation
- PMID: 12087128
- PMCID: PMC123179
- DOI: 10.1073/pnas.142298599
Role of the short isoform of myosin light chain kinase in the contraction of cultured smooth muscle cells as examined by its down-regulation
Abstract
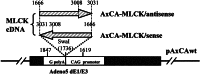
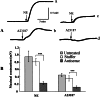
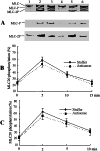
GbaSM-4 cells, smooth muscle cells derived from brain basilar artery, which express both 210-kDa long and 130-kDa short isoforms of myosin light chain kinase (MLCK), were infected with an adenovirus vector carrying a 1.4-kb catalytic portion of MLCK-cDNA in an antisense orientation. Western blot analysis showed that the expression of short MLCK was depressed without affecting long MLCK expression. The contraction of the down-regulated cells was measured by the cell-populated collagen-fiber method. The tension development after stimulation with norepinephrine or was depressed. The additional infection of the down-regulated cells with the adenovirus construct containing the same insert in a sense direction rescued not only the short MLCK expression but also contraction, confirming the physiological role of short MLCK in the contraction. To examine the role of long MLCK in the residual contraction persisting in the short MLCK-deficient cells, long MLCK was further down-regulated by increasing the multiplicity of infection of the antisense construct. The additional down-regulation of long MLCK expression, however, did not alter the residual contraction, ruling out the involvement of long MLCK in the contractile activity. Further, in the cells where short MLCK was down-regulated specifically, the extent of phosphorylation of 20-kDa myosin light chain (MLC20) after the agonist stimulation was not affected. This finding suggests that there are additional factors to MLC20 phosphorylation that contribute to regulate smooth muscle contraction.
Figures


References
-
- Kamm K E, Stull J T. Annu Rev Pharmacol Toxicol. 1985;25:593–620. - PubMed
-
- Ye L-H, Hayakawa K, Kishi H, Imamura M, Nakamura A, Okagaki T, Takagi T, Iwata A, Tanaka T, Kohama K. J Biol Chem. 1997;272:32182–32189. - PubMed
-
- Smith L, Su X, Lin P-J, Zhi G, Stull J T. J Biol Chem. 1999;274:29433–29438. - PubMed
-
- Ito M, Dabrowska R, Guerriero V, Jr, Hartshorne D J. J Biol Chem. 1989;264:13971–13974. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

