Conformational change of Escherichia coli initiator methionyl-tRNA(fMet) upon binding to methionyl-tRNA formyl transferase
- PMID: 12087168
- PMCID: PMC117066
- DOI: 10.1093/nar/gkf411
Conformational change of Escherichia coli initiator methionyl-tRNA(fMet) upon binding to methionyl-tRNA formyl transferase
Abstract
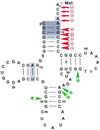
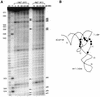
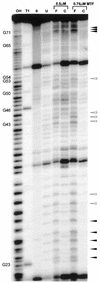
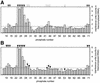
The specific formylation of initiator methionyl-tRNA (Met-tRNA) by methionyl-tRNA formyltransferase (MTF) is important for the initiation of protein synthesis in Escherichia coli. The determinants for formylation are located in the acceptor stem and in the dihydrouridine (D) stem of the initiator tRNA (tRNA(fMet)). Here, we have used ethylation interference analysis to study the interactions between the Met-tRNA(fMet) and MTF in solution. We have identified three clusters of phosphates in the tRNA that, when ethylated, interfere with binding of MTF. Interference due to ethylation of phosphates in the acceptor stem and in the D stem is most likely due to the close proximity of the protein as seen in the crystal structure of the MTF.fMet-tRNA(fMet) complex. The third cluster of phosphates, whose ethylation interferes with binding of MTF, is dispersed along the anticodon stem, which is distal to the sites of tRNA protein contacts. Interestingly, these latter positions correspond to sites of increased cleavages by RNase V1 in RNA footprinting experiments. Together, these results suggest that in addition to the protein, which binds to the substrate tRNA in an induced fit mechanism, the tRNA also undergoes induced structural changes during its binding to MTF.
Figures

References
-
- Kozak M. (1999) Initiation of translation in prokaryotes and eukaryotes. Gene, 234, 187–208. - PubMed
-
- Varshney U., Lee,C.P., Seong,B.L. and RajBhandary,U.L. (1991) Mutants of initiator tRNA that function both as initiators and elongators. J. Biol. Chem., 266, 18018–18024. - PubMed
-
- Sundari R.M., Stringer,E.A., Schulman,L.H. and Maitra,U. (1976) Interaction of bacterial initiation factor 2 with initiator tRNA. J. Biol. Chem., 251, 3338–3345. - PubMed
-
- Nissen P., Kjeldgaard,M., Thirup,S., Polekhina,G., Reshetnikova,L., Clark,B.F. and Nyborg,J. (1995) Crystal structure of the ternary complex of Phe-tRNAPhe, EF-Tu and a GTP analog. Science, 270, 1464–1472. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

