Effects of double-strand break repair proteins on vertebrate telomere structure
- PMID: 12087170
- PMCID: PMC117051
- DOI: 10.1093/nar/gkf396
Effects of double-strand break repair proteins on vertebrate telomere structure
Abstract
Although telomeres are not recognized as double-strand breaks (DSBs), some DSB repair proteins are present at telomeres and are required for telomere maintenance. To learn more about the telomeric function of proteins from the homologous recombination (HR) and non-homologous end joining pathways (NHEJ), we have screened a panel of chicken DT40 knockout cell lines for changes in telomere structure. In contrast to what has been observed in Ku-deficient mice, we found that Ku70 disruption did not result in telomere-telomere fusions and had no effect on telomere length or the structure of the telomeric G-strand overhang. G-overhang length was increased by Rad51 disruption but unchanged by disruption of DNA-PKcs, Mre11, Rad52, Rad54, XRCC2 or XRCC3. The effect of Rad51 depletion was unexpected because gross alterations in telomere structure have not been detected in yeast HR mutants. Thus, our results indicate that Rad51 has a previously undiscovered function at vertebrate telomeres. They also indicate that Mre11 is not required to generate G-overhangs. Although Mre11 has been implicated in overhang generation, overhang structure had not previously been examined in Mre11-deficient cells. Overall our findings indicate that there are significant species-specific differences in the telomeric function of DSB repair proteins.
Figures

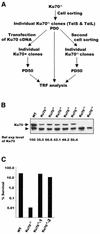

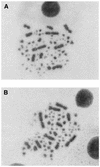
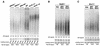
Similar articles
-
Mammalian Ku86 protein prevents telomeric fusions independently of the length of TTAGGG repeats and the G-strand overhang.EMBO Rep. 2000 Sep;1(3):244-52. doi: 10.1093/embo-reports/kvd051. EMBO Rep. 2000. PMID: 11256607 Free PMC article.
-
Components of the Ku-dependent non-homologous end-joining pathway are involved in telomeric length maintenance and telomeric silencing.EMBO J. 1998 Mar 16;17(6):1819-28. doi: 10.1093/emboj/17.6.1819. EMBO J. 1998. PMID: 9501103 Free PMC article.
-
Multiple roles for MRE11 at uncapped telomeres.Nature. 2009 Aug 13;460(7257):914-8. doi: 10.1038/nature08196. Epub 2009 Jul 26. Nature. 2009. PMID: 19633651 Free PMC article.
-
Ku, a DNA repair protein with multiple cellular functions?Mutat Res. 1999 May 14;434(1):3-15. doi: 10.1016/s0921-8777(99)00006-3. Mutat Res. 1999. PMID: 10377944 Review.
-
Mechanisms of DNA double strand break repair and chromosome aberration formation.Cytogenet Genome Res. 2004;104(1-4):14-20. doi: 10.1159/000077461. Cytogenet Genome Res. 2004. PMID: 15162010 Review.
Cited by
-
Biosemantics guided gene expression profiling of Sjögren's syndrome: a comparative analysis with systemic lupus erythematosus and rheumatoid arthritis.Arthritis Res Ther. 2017 Aug 17;19(1):192. doi: 10.1186/s13075-017-1400-3. Arthritis Res Ther. 2017. PMID: 28818099 Free PMC article.
-
Human ovarian surface epithelial cells immortalized with hTERT maintain functional pRb and p53 expression.Cell Prolif. 2007 Oct;40(5):780-94. doi: 10.1111/j.1365-2184.2007.00462.x. Cell Prolif. 2007. PMID: 17877616 Free PMC article.
-
DNA repair polymorphisms and outcome of chemotherapy for acute myelogenous leukemia: a report from the Children's Oncology Group.Leukemia. 2008 Feb;22(2):265-72. doi: 10.1038/sj.leu.2405000. Epub 2007 Nov 22. Leukemia. 2008. PMID: 18033323 Free PMC article.
-
Identifying Compounds with Genotoxicity Potential Using Tox21 High-Throughput Screening Assays.Chem Res Toxicol. 2019 Jul 15;32(7):1384-1401. doi: 10.1021/acs.chemrestox.9b00053. Epub 2019 Jun 18. Chem Res Toxicol. 2019. PMID: 31243984 Free PMC article.
-
Ovate family protein 1 as a plant Ku70 interacting protein involving in DNA double-strand break repair.Plant Mol Biol. 2010 Nov;74(4-5):453-66. doi: 10.1007/s11103-010-9685-5. Epub 2010 Sep 16. Plant Mol Biol. 2010. PMID: 20844935
References
-
- Karran P. (2000) DNA double strand break repair in mammalian cells. Curr. Opin. Genet. Dev., 10, 144–150. - PubMed
-
- Khanna K.K. and Jackson,S.P. (2001) DNA double-strand breaks: signaling, repair and the cancer connection. Nature Genet., 27, 247–254. - PubMed
-
- Lundblad V. (2000) DNA ends: maintenance of chromosome termini versus repair of double strand breaks. Mutat. Res., 451, 227–240. - PubMed
-
- Greider C.W. (1996) Telomere length regulation. Annu. Rev. Biochem., 65, 337–365. - PubMed
-
- Griffith J.D., Comeau,L., Rosenfield,S., Stansel,R.M., Bianchi,A., Moss,H. and de Lange,T. (1999) Mammalian telomeres end in a large duplex loop. Cell, 97, 503–514. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

