Synthesis and application of charge-modified dye-labeled dideoxynucleoside-5'-triphosphates to 'direct-load' DNA sequencing
- PMID: 12087172
- PMCID: PMC117042
- DOI: 10.1093/nar/gkf387
Synthesis and application of charge-modified dye-labeled dideoxynucleoside-5'-triphosphates to 'direct-load' DNA sequencing
Abstract
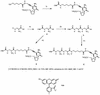
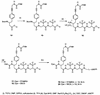
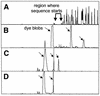
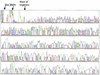
A novel series of charge-modified, dye-labeled 2',3'-dideoxynucleoside-triphosphate terminators were synthesized and evaluated as reagents for DNA sequencing. These terminators possess an advantage over existing reagents in that no purification is required to remove unreacted nucleotide or associated breakdown products prior to electrophoretic separation of the sequencing fragments. This obviates the need for a time consuming post-reaction work up, allowing direct loading of DNA sequencing reaction mixtures onto a slab gel. Thermo Sequenase II DNA polymerase poorly incorporates the charge-modified terminators compared with regular dye-labeled terminators. However, extending the linker arm between dye and nucleotide and using a mutant form of a related DNA polymerase can in part mitigate the decrease in substrate efficiency. We also present evidence that these charge-modified terminators can relieve gel compression artefacts when used with dGTP in sequencing reactions.
Figures



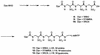
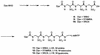




Similar articles
-
Efficient incorporation of positively charged 2', 3'-dideoxynucleoside-5'-triphosphates by DNA polymerases and their application in 'direct-load' DNA sequencing.Nucleic Acids Res. 2003 Aug 15;31(16):4769-78. doi: 10.1093/nar/gkg669. Nucleic Acids Res. 2003. PMID: 12907718 Free PMC article.
-
Novel cyanine dye-labeled dideoxynucleoside triphosphates for DNA sequencing.Bioconjug Chem. 2002 Jul-Aug;13(4):699-706. doi: 10.1021/bc015522y. Bioconjug Chem. 2002. PMID: 12121123
-
New protocols for DNA sequencing with dye terminators.DNA Seq. 1992;3(1):61-4. doi: 10.3109/10425179209039697. DNA Seq. 1992. PMID: 1457810
-
Automated fluorescent DNA sequencing on the ABI PRISM 377.Methods Mol Biol. 2001;167:119-52. doi: 10.1385/1-59259-113-2:119. Methods Mol Biol. 2001. PMID: 11265312 Review. No abstract available.
-
PCR and DNA sequencing.Biotechniques. 1989 Jul-Aug;7(7):700-8. Biotechniques. 1989. PMID: 2698653 Review.
Cited by
-
Efficient incorporation of positively charged 2', 3'-dideoxynucleoside-5'-triphosphates by DNA polymerases and their application in 'direct-load' DNA sequencing.Nucleic Acids Res. 2003 Aug 15;31(16):4769-78. doi: 10.1093/nar/gkg669. Nucleic Acids Res. 2003. PMID: 12907718 Free PMC article.
-
Molecular methods in cancer diagnostics: a short review.Ann Med. 2024 Dec;56(1):2353893. doi: 10.1080/07853890.2024.2353893. Epub 2024 May 16. Ann Med. 2024. PMID: 38753424 Free PMC article. Review.
-
Comparative Study of Novel Fluorescent Cyanine Nucleotides: Hybridization Analysis of Labeled PCR Products Using a Biochip.J Fluoresc. 2017 Nov;27(6):2001-2016. doi: 10.1007/s10895-017-2139-6. Epub 2017 Jul 28. J Fluoresc. 2017. PMID: 28752470
References
-
- Smith L.M., Sanders,J.Z., Kaiser,R.J., Hughes,P., Doss,C., Connell,C.R., Heiner,C., Kents,S.B.H. and Hood,L. (1986) Fluorescence detection in automated DNA sequencing. Nature, 321, 674–678. - PubMed
-
- Prober G.M., Trainor,G.L., Dam,R.J., Hobbs,F.W., Robertson,C.W., Zagursky,R.J., Cocuzza,A.J., Jensen,M.A. and Baumeister,K. (1987) A system for rapid DNA sequencing with fluorescent chain-terminating dideoxynucleotides. Science 238, 336–341. - PubMed
-
- Swedlow H., Zhang,J.Z., Chen,D.Y., Harke,H.R., Grey,R., Wu,S. and Dovichi,N. (1991) Three DNA sequencing methods based on capillary gel electrophoresis and laser-induced fluorescence. Anal. Chem., 63, 2835–2841. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

