Pentamidine inhibits catalytic activity of group I intron Ca.LSU by altering RNA folding
- PMID: 12087182
- PMCID: PMC117049
- DOI: 10.1093/nar/gkf394
Pentamidine inhibits catalytic activity of group I intron Ca.LSU by altering RNA folding
Abstract
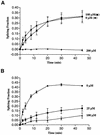
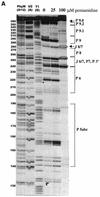
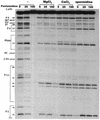
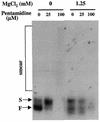
The antimicrobial agent pentamidine inhibits the self-splicing of the group I intron Ca.LSU from the transcripts of the 26S rRNA gene of Candida albicans, but the mechanism of pentamidine inhibition is not clear. We show that preincubation of the ribozyme with pentamidine enhances the inhibitory effect of the drug and alters the folding of the ribozyme in a pattern varying with drug concentration. Pentamidine at 25 microM prevents formation of the catalytically active F band conformation of the precursor RNA and alters the ribonuclease T1 cleavage pattern of Ca.LSU RNA. The effects on cleavage suggest that pentamidine mainly binds to specific sites in or near asymmetric loops of helices P2 and P2.1 on the ribozyme, as well as to the tetraloop of P9.2 and the loosely paired helix P9, resulting in an altered structure of helix P7, which contains the active site. Positively charged molecules antagonize pentamidine inhibition of catalysis and relieve the drug effect on ribozyme folding, suggesting that pentamidine binds to a magnesium binding site(s) of the ribozyme to exert its inhibitory effect.
Figures






Similar articles
-
Folding of the group I intron ribozyme from the 26S rRNA gene of Candida albicans.Nucleic Acids Res. 2001 Jun 15;29(12):2644-53. doi: 10.1093/nar/29.12.2644. Nucleic Acids Res. 2001. PMID: 11410674 Free PMC article.
-
Pentamidine inhibition of group I intron splicing in Candida albicans correlates with growth inhibition.Antimicrob Agents Chemother. 2000 Apr;44(4):958-66. doi: 10.1128/AAC.44.4.958-966.2000. Antimicrob Agents Chemother. 2000. PMID: 10722497 Free PMC article.
-
Concerted folding of a Candida ribozyme into the catalytically active structure posterior to a rapid RNA compaction.Nucleic Acids Res. 2003 Jul 15;31(14):3901-8. doi: 10.1093/nar/gkg455. Nucleic Acids Res. 2003. PMID: 12853605 Free PMC article.
-
Productive folding to the native state by a group II intron ribozyme.J Mol Biol. 2002 Jan 18;315(3):297-310. doi: 10.1006/jmbi.2001.5233. J Mol Biol. 2002. PMID: 11786013
-
The ribozyme core of group II introns: a structure in want of partners.Trends Biochem Sci. 2009 Apr;34(4):189-99. doi: 10.1016/j.tibs.2008.12.007. Epub 2009 Mar 18. Trends Biochem Sci. 2009. PMID: 19299141 Review.
Cited by
-
Polymorphism in Mitochondrial Group I Introns among Cryptococcus neoformans and Cryptococcus gattii Genotypes and Its Association with Drug Susceptibility.Front Microbiol. 2018 Feb 6;9:86. doi: 10.3389/fmicb.2018.00086. eCollection 2018. Front Microbiol. 2018. PMID: 29467729 Free PMC article.
-
Evaluating target silencing by short hairpin RNA mediated by the group I intron in cultured mammalian cells.BMC Biotechnol. 2011 Jul 25;11:79. doi: 10.1186/1472-6750-11-79. BMC Biotechnol. 2011. PMID: 21781346 Free PMC article.
-
Pentamidine reverses the splicing defects associated with myotonic dystrophy.Proc Natl Acad Sci U S A. 2009 Nov 3;106(44):18551-6. doi: 10.1073/pnas.0903234106. Epub 2009 Oct 12. Proc Natl Acad Sci U S A. 2009. PMID: 19822739 Free PMC article.
-
Regulation of MALAT1 triple helix stability and in vitro degradation by diphenylfurans.Nucleic Acids Res. 2020 Aug 20;48(14):7653-7664. doi: 10.1093/nar/gkaa585. Nucleic Acids Res. 2020. PMID: 32667657 Free PMC article.
-
Pentamidine inhibits Coxiella burnetii growth and 23S rRNA intron splicing in vitro.Int J Antimicrob Agents. 2010 Oct;36(4):380-2. doi: 10.1016/j.ijantimicag.2010.05.017. Int J Antimicrob Agents. 2010. PMID: 20599360 Free PMC article.
References
-
- Sands M., Kron,M.A. and Brown,R.B. (1985) Pentamidine: a review. Rev. Infect. Dis., 7, 625–634. - PubMed
-
- Van Voorhis W.C. (1990) Therapy and prophylaxis of systemic protozoan infections. Drugs, 40, 176–202. - PubMed
-
- Grady R.W., Blobstein,S.H., Meshnick,S.R., Ulrich,P.C., Cerami,A., Amirmoazzami,J. and Hodnett,E.M. (1984) The in vitro trypanocidal activity of N-substituted p-benzoquinone imines: assessment of biochemical structure-activity relationships using the Hansch approach. J. Cell. Biochem., 25, 15–29. - PubMed
-
- Dykstra C.C. and Tidwell,R.R. (1991) Inhibition of topoisomerases from Pneumocystis carinii by aromatic dicationic molecules. J. Protozool., 38, 78S–81S. - PubMed

