Duration of adjuvant chemotherapy for breast cancer: a joint analysis of two randomised trials investigating three versus six courses of CMF
- PMID: 12087454
- PMCID: PMC2375405
- DOI: 10.1038/sj.bjc.6600334
Duration of adjuvant chemotherapy for breast cancer: a joint analysis of two randomised trials investigating three versus six courses of CMF
Abstract
Cyclophosphamide, methotrexate and fluorouracil adjuvant combination chemotherapy for breast cancer is currently used for the duration of six monthly courses. We performed a joint analysis of two studies on the duration of adjuvant cyclophosphamide, methotrexate and fluorouracil in patients with node-positive breast cancer to investigate whether three courses of cyclophosphamide, methotrexate and fluorouracil might suffice. The International Breast Cancer Study Group Trial VI randomly assigned 735 pre- and perimenopausal patients to receive 'classical' cyclophosphamide, methotrexate and fluorouracil for three consecutive cycles, or the same chemotherapy for six consecutive cycles. The German Breast Cancer Study Group randomised 289 patients to receive either three or six cycles of i.v. cyclophosphamide, methotrexate and fluorouracil day 1, 8. Treatment effects were estimated using Cox regression analysis stratified by clinical trial without further adjustment for covariates. The 5-year disease-free survival per cents (+/-s.e.) were 54+/-2% for three cycles and 55+/-2% for six cycles (n=1024; risk ratio (risk ratio: CMFx3/CMFx6), 1.00; 95% confidence interval, 0.85 to 1.18; P=0.99). Use of three rather than six cycles was demonstrated to be adequate in both studies for patients at least 40-years-old with oestrogen-receptor-positive tumours (n=594; risk ratio, 0.86; 95% confidence interval, 0.68 to 1.08; P=0.19). In fact, results slightly favoured three cycles over six for this subgroup, and the 95% confidence interval excluded an adverse effect of more than 2% with respect to absolute 5-year survival. In contrast, three cycles appeared to be possibly inferior to six cycles for women less than 40-years-old (n=190; risk ratio, 1.25; 95% confidence interval, 0.87 to 1.80; P=0.22) and for women with oestrogen-receptor-negative tumours (n=302; risk ratio, 1.15; 95% confidence interval, 0.85 to 1.57; P=0.37). Thus, three initial cycles of adjuvant cyclophosphamide, methotrexate and fluorouracil chemotherapy were as effective as six cycles for older patients (40-years-old) with oestrogen-receptor-positive tumours, while six cycles of adjuvant cyclophosphamide, methotrexate and fluorouracil might still be required for other cohorts. Because endocrine therapy with tamoxifen and GnRH analogues is now available for younger women with oestrogen-receptor-positive tumours, the need for six cycles of cyclophosphamide, methotrexate and fluorouracil is unclear and requires further investigation.
Copyright 2002 Cancer Research UK
Figures
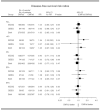



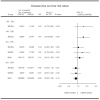
Similar articles
-
Duration and reintroduction of adjuvant chemotherapy for node-positive premenopausal breast cancer patients.J Clin Oncol. 1996 Jun;14(6):1885-94. doi: 10.1200/JCO.1996.14.6.1885. J Clin Oncol. 1996. PMID: 8656257 Clinical Trial.
-
Adjuvant chemotherapy followed by goserelin versus either modality alone for premenopausal lymph node-negative breast cancer: a randomized trial.J Natl Cancer Inst. 2003 Dec 17;95(24):1833-46. doi: 10.1093/jnci/djg119. J Natl Cancer Inst. 2003. PMID: 14679153 Clinical Trial.
-
Dose-response effect of adjuvant cyclophosphamide, methotrexate, 5-fluorouracil (CMF) in node-positive breast cancer. International Breast Cancer Study Group.Eur J Cancer. 1998 Oct;34(11):1693-700. doi: 10.1016/s0959-8049(98)00209-3. Eur J Cancer. 1998. PMID: 9893654
-
Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer?Lancet. 2000 May 27;355(9218):1869-74. doi: 10.1016/s0140-6736(00)02292-3. Lancet. 2000. PMID: 10866443 Review.
-
Doxorubicin in combination with fluorouracil and cyclophosphamide (i.v. FAC regimen, day 1, 21) versus methotrexate in combination with fluorouracil and cyclophosphamide (i.v. CMF regimen, day 1, 21) as adjuvant chemotherapy for operable breast cancer: a study by the GEICAM group.Ann Oncol. 2003 Jun;14(6):833-42. doi: 10.1093/annonc/mdg260. Ann Oncol. 2003. PMID: 12796019 Review.
Cited by
-
Differential efficacy of three cycles of CMF followed by tamoxifen in patients with ER-positive and ER-negative tumors: long-term follow up on IBCSG Trial IX.Ann Oncol. 2011 Sep;22(9):1981-1987. doi: 10.1093/annonc/mdq754. Epub 2011 Jan 31. Ann Oncol. 2011. PMID: 21282282 Free PMC article. Clinical Trial.
-
Efficacy of adjuvant chemotherapy with carboplatin for early triple negative breast cancer: a single center experience.Oncotarget. 2017 May 23;8(43):75617-75626. doi: 10.18632/oncotarget.18118. eCollection 2017 Sep 26. Oncotarget. 2017. PMID: 29088896 Free PMC article.
-
The effect of endocrine responsiveness on high-risk breast cancer treated with dose-intensive chemotherapy: results of International Breast Cancer Study Group Trial 15-95 after prolonged follow-up.Ann Oncol. 2009 Aug;20(8):1344-51. doi: 10.1093/annonc/mdp024. Epub 2009 May 25. Ann Oncol. 2009. PMID: 19468030 Free PMC article. Clinical Trial.
-
Endocrine-responsive lobular carcinoma of the breast: features associated with risk of late distant recurrence.Breast Cancer Res. 2019 Dec 30;21(1):153. doi: 10.1186/s13058-019-1234-9. Breast Cancer Res. 2019. PMID: 31888717 Free PMC article.
-
Mixture survival trees for cancer risk classification.Lifetime Data Anal. 2022 Jul;28(3):356-379. doi: 10.1007/s10985-022-09552-w. Epub 2022 Apr 29. Lifetime Data Anal. 2022. PMID: 35486260 Free PMC article.
References
-
- AebiSGelberSCastiglione-GertschMGelberRDCollinsJThurlimannBRudenstamCMLindtnerJCrivellariDCortes-FunesHSimonciniEWernerIDCoatesASGoldhirschAfor the International Breast Cancer Study Group (IBCSG)2000Is chemotherapy alone adequate for young women with oestrogen-receptor-positive breast cancer? Lancet 35518691874 - PubMed
-
- BoccardoFRubagottiAAmorosoDMesitiMRomeoDSismondiPGiaiFGentaFPaciniPDistanteVBolognesiAAldrighettiDFarrisA2000Cyclophosphamide, methotrexate, and fluorouracil versus tamoxifen plus ovarian suppression as adjuvant treatment of estrogen receptor-positive pre-perimenopausal breast cancer patients: results of the Italian Breast Cancer Adjuvant Study Group O2 Randomized Trial J Clin Oncol 1827182727 - PubMed
-
- BonadonnaGValagussaPZambettiMBuzzoniR1987Milan adjuvant trials for stage I-II breast cancerInAdjuvant Therapy of Cancer VSalmon SE (ed)pp211221.Orlando: Grune & Stratton
-
- BonettiMGelberRD2000A graphical method to assess treatment-covariate interactions using the Cox model on subsets of the data Stat in Med 1925952609 - PubMed
-
- BonettiMGelberRD2001STEPP: Subsets analyses and patterns of treatment-covariate interactions in clinical trialsDana-Farber Cancer Institute Department of Biostatistical Sciences Technical Report Number 583Z
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

