CD10 is a marker for cycling cells with propensity to apoptosis in childhood ALL
- PMID: 12087466
- PMCID: PMC2375395
- DOI: 10.1038/sj.bjc.6600329
CD10 is a marker for cycling cells with propensity to apoptosis in childhood ALL
Abstract
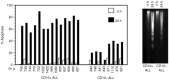
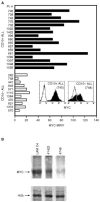
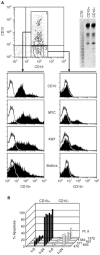
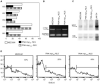
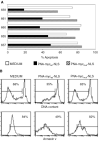
CD10 constitutes a favourable prognostic marker for childhood acute lymphoblastic leukaemia. Since correlations between CD10, cell cycle and apoptotic abilities were demonstrated in various cell types, we investigated whether differences existed in the cycling/apoptotic abilities of CD10-positive and CD10-negative B acute lymphoblastic leukaemia cells. Twenty-eight cases of childhood acute lymphoblastic leukaemia (mean age of 6.8 years) were subdivided into two groups according to high (17 cases, 93.2+/-4.5%, MRFI 211+/-82 CD10-positive cells) or low (11 cases, 11.5+/-6.2%, MRFI 10+/-7 CD10-negative cells) expression of CD10. CD10-positive acute lymphoblastic leukaemia cells were cycling cells with elevated c-myc levels and propensity to apoptosis, whereas CD10-negative acute lymphoblastic leukaemia cells had lower cycling capacities and c-myc levels, and were resistant to apoptosis in vitro. A close correlation between all these properties was demonstrated by the observations that the few CD10-positive cells found in the CD10-negative acute lymphoblastic leukaemia group displayed elevated c-myc and cycling capacities and were apoptosis prone. Moreover, exposure of CD10-positive acute lymphoblastic leukaemia B cells to a peptide nucleic acid anti-gene specific for the second exon of c-myc caused inhibition of c-myc expression and reduced cell cycling and apoptotic abilities as well as decreased CD10 expression.
Copyright 2002 Cancer Research UK
Figures



References
-
- ArmitagePBerryG1987Statistical Methods in Medical Research,London: Blackwell Scientific Publications
-
- BahramFvon der LehrNCetinkayaCLarssonLG2000c-Myc hot spot mutations in lymphomas result in inefficient ubiquitination and decreased proteasome-mediated turnover Blood 1521042110 - PubMed
-
- BennettJCatovskyDDanielMTFlandrinGGaltonDAGralnickHRSultanC1981The morphological classification of acute lymphoblastic leukaemia: concordance among observers and clinical correlations Br J Haematol 47553561 - PubMed
-
- BissonnetteREcheverriFMahboubiAGreenDR1992Apoptotic cell death induced by c-myc is inhibited by bcl-2 Nature 359552554 - PubMed
-
- BorkhardtACazzanigaGViehmannSValsecchiMGLudwigWDBurciLMangioniSSchrappeMRiehmHLampertFBassoGMaseraGHarbottJBiondiA1997Incidence and clinical relevance of TEL/AML1 fusion genes in children with acute lymphoblastic leukemia enrolled in the German and Italian multicenter therapy trials. Associazione Italiana Ematologia Oncologia Pediatrica and the Berlin-Frankfurt-Munster Study Group Blood 90571577 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

