Periodate-treated, non-anticoagulant heparin-carrying polystyrene (NAC-HCPS) affects angiogenesis and inhibits subcutaneous induced tumour growth and metastasis to the lung
- PMID: 12087470
- PMCID: PMC2375397
- DOI: 10.1038/sj.bjc.6600307
Periodate-treated, non-anticoagulant heparin-carrying polystyrene (NAC-HCPS) affects angiogenesis and inhibits subcutaneous induced tumour growth and metastasis to the lung
Abstract
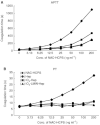
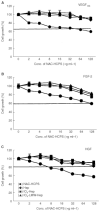
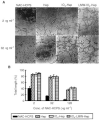
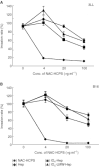
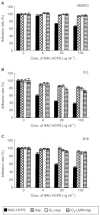
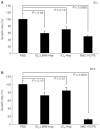
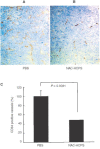
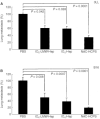
Periodate-treated, non-anticoagulant heparin-carrying polystyrene consists of about ten periodate-oxidized, alkaline-degraded low molecular weight-heparin chains linked to a polystyrene core and has a markedly lower anti-coagulant activity than heparin. In this study, we evaluated the effect of non-anticoagulant heparin-carrying polystyrene on tumour growth and metastasis. Non-anticoagulant heparin-carrying polystyrene has a higher activity to inhibit vascular endothelial growth factor-165-, fibroblast growth factor-2- or hepatocyte growth factor-induced human microvascular endothelial cell growth than heparin, ten periodate-oxidized-heparin and ten periodate-oxidized-low molecular weight-heparin, which is probably due to the heparin-clustering effect of non-anticoagulant heparin-carrying polystyrene. Non-anticoagulant heparin-carrying polystyrene inhibited human microvascular endothelial cell, B16 melanoma and Lewis lung cancer cell adhesion to Matrigel-coated plates. Non-anticoagulant heparin-carrying polystyrene also showed strong inhibitory activities in the tubular formation of endothelial cells on Matrigel and B16-melanoma and Lewis lung cancer cell invasion in a Matrigel-coated chamber assay. In vivo studies showed that growth of subcutaneous induced tumours and lung metastasis of B16-melanoma and Lewis lung cancer cells were more effectively inhibited by non-anticoagulant heparin-carrying polystyrene than ten periodate-oxidized-heparin and ten periodate-oxidized-low molecular weight-heparin. Furthermore, non-anticoagulant heparin-carrying polystyrene markedly reduced the number of CD34-positive vessels in subcutaneous Lewis lung cancer tumours, indicating a strong inhibition of angiogenesis. These results suggest that non-anticoagulant heparin-carrying polystyrene has an inhibitory activity on angiogenesis and tumour invasion and may be very useful in cancer therapy.
Copyright 2002 Cancer Research UK
Figures

References
-
- AlbiniAIwamotoYKleinmanHKMartinGRAarosonSAKozlowskiJMMcEwanRN1987A rapid in vitro assay for quantitating the invasion potential of tumour cells Cancer Res 4732393245 - PubMed
-
- BasbaumCBWerbZ1996Focalized proteolysis: Spatial and temporal regulation of extracellular matrix degradation at the cell surface Curr Opin Cell Biol 8731738 - PubMed
-
- CollenASmorenburgSMPetersELupuFKoolwijkPVon NoordenCHinsberghVWM2000Unfractionated and low molecular weight heparin affect fibrin structure and angiogenesis in vitro Cancer Res 6061926200 - PubMed
-
- ConradHEGuoY1991Structural analysis of periodate-oxidized heparinInHeparin And Related Polysaccharides, Advances in Experimental Medicine and Biology 313Lane DA, Bjork I, Lindahl U (eds)pp3136New York: Plenum Publishing - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

